电池和储能
COMMON CHALLENGES
Modern energy storage technologies, such as lithium-ion batteries, are used in more and more applications every day. The proliferation of energy storage technologies also drives the demand for materials with improved performance in key metrics such as power and energy density, Coulombic efficiency, cycle lifetime, safety, and stability.
Researchers can develop new materials or chemistries to improve on key performance metrics by understanding how they are affected by fundamental aspects like crystal structure, diffusion rates, electron transport, and other electrochemical dynamics. Characterizing materials properties, such as atomic structure and elemental distribution, is the first step towards elucidating these structure-property relationships.
INNOVATIVE TECHNIQUES
Several techniques are available to better characterize and understand crucial structure-property relationships in energy storage materials.
|
Study morphology and crystal structure at varying (electro)chemical states.
Apply 4D STEM techniques for virtual imaging and diffraction, along with mapping crystalline strain.
|
Apply various stimuli and observe phase transformations and changing elemental distributions in real-time.
Use low-dose techniques to study the dynamic phenomena and sensitive materials without beam effects. |
|
Identify and quantify light elements such as lithium and oxygen in your specimen.
Determine the oxidation state of important elements, such as transition metals.
Map the elemental distribution of your specimen down to the atomic scale.
|
Family of imaging techniques to enhance, map, and quantify elements and chemicals in an image, with nanometer resolution.
Utilize alongside either in-situ and ex-situ experiments.
|
|
Specimen preparation
Prepare cross-sections of materials and devices to study structural and composition changes on a larger scale.
Perform final cleaning on FIB cross-section specimens to achieve damage-free surfaces.
Deposit various coatings with nanometer control.
|
Specimen handling
Preserve specimen integrity with controlled transfer from an inert environment to the SEM or TEM.
Prevent unwanted oxidation or deterioration of air-sensitive materials during handling.
Protect and preserve delicate structures with imaging and analysis at cryogenic temperatures.
|
|
Analyze, quantify, and map the distribution of elements in a specimen.
|
Examine crystallographic orientation and texture in materials.
|
Visit solar, utilities, and environment and semiconductor materials and devices to learn about related applications.
ENABLING RESULTS
Many initial studies on new energy storage materials focus on characterizing material properties in the pristine state, followed by determining property changes after cycling to different states of charge, e.g., ex-situ characterization. Electron microscopy provides various imaging and elemental analysis techniques with micro-scale to atomic-scale spatial resolution.
The choice of scanning electron microscopy (SEM) versus transmission electron microscopy (TEM) techniques depends on the nature of the specimen (e.g., bulk, powder, nanomaterial) and the desired analysis.
Common SEM characterization:
- Surface imaging of an electrode over a large region of interest
- Imaging cross-sections of an electrode or battery cell
- Elemental analysis over a large region of interest with EDS
- Grain structure and orientation analysis of cross-sections with EBSD
Visit EDAX.com for more information on EDS and EBSD products and applications.
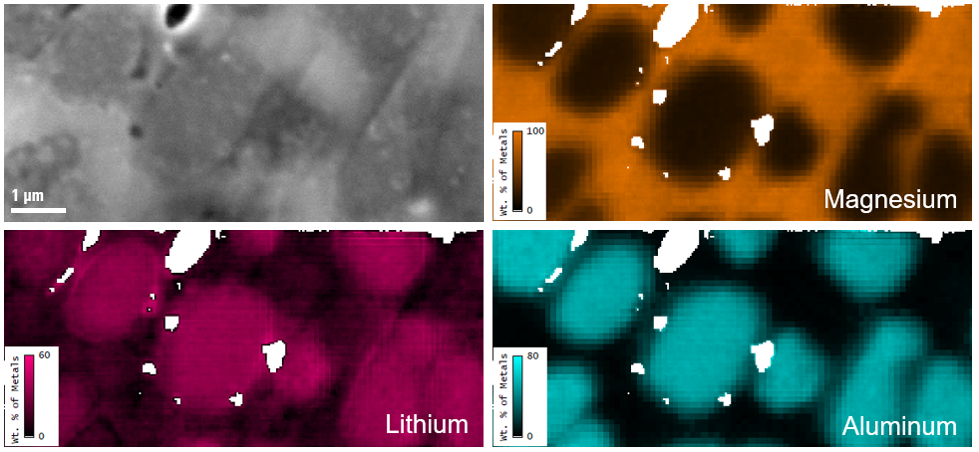

Common TEM characterization:
- Nanometer and atomic-scale imaging of materials
- Crystal structure determination with electron diffraction techniques (e.g., selected area electron diffraction, diffraction tomography, 4D STEM)
- Elemental analysis with EDS and EELS
- Chemical state analysis of individual elements with EELS
- Imaging of elemental or chemical state distribution with EFTEM
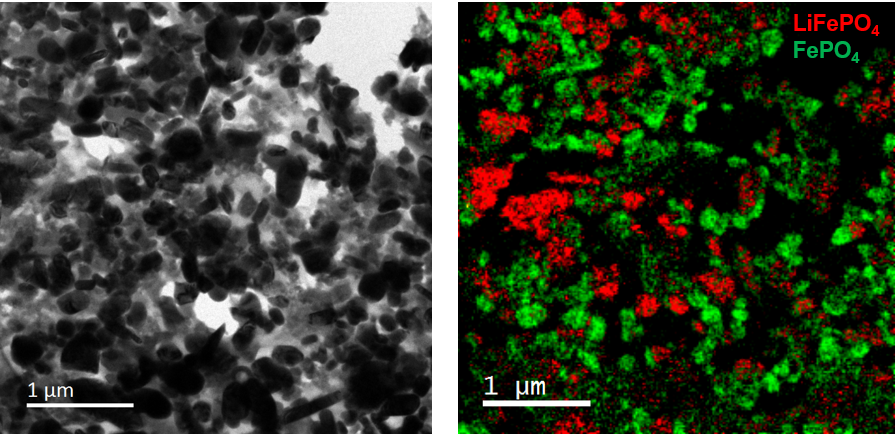

Energy storage materials exist in constantly evolving (electro)chemical states as they repeatedly convert chemical energy into electrical energy and vice-versa. Understanding this dynamic behavior is key to developing high-performance materials. Studies of this type are accomplished in an electron microscope by imaging a specimen or conducting chemical analysis while also providing an electrochemical stimulus to the specimen, e.g., in-situ microscopy.
There are various setups for in-situ TEM experiments on energy storage materials. Most experiments choose to study an individual element of an entire energy storage device, e.g., the cathode of a battery or a particular half-cell reaction. Simpler experiments forgo using a liquid electrolyte in the in-situ experiment to avoid the complication and still observe dynamic phenomena, at the cost of being a degree removed from actual battery operation conditions. More complex experiments use a specialized TEM specimen holder to bring a full liquid electrolyte cell into the TEM.
In either case, in-situ experiments present several challenges to the microscopist in addition to the basic experimental design and setup:
- Working under low-dose conditions to prevent specimen damage and other beam-induced artifacts
- Acquiring data with the required spatial and temporal resolution to study the reactions of interest properly
- Managing the large amount of data generated (in many cases, several TBs of video data) during a single in-situ experiment
Gatan’s portfolio of in-situ products greatly aids microscopists in addressing and managing all the challenges that come with in-situ experiments on energy storage materials.
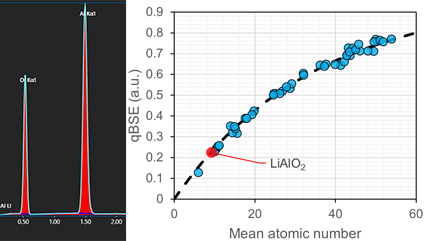
Application: 使用成分差异法对锂元素进行定量表征
Gatan’s OnPoint™ detector and EDAX’s Octane Elite Super EDS system were used in combination to determine the lithium concentration in a stoichiometric lithium oxide sample.
Read the full experiment brief
Visit the OnPoint product page to find other examples of low-dose imaging via real-time electron counting.
Application: Imaging a lithium metal battery solid electrolyte interphase
Developing better batteries is critical for advancing a number of technologies for energy and the environment, and lithium (Li) metal is an attractive anode material based on its fundamental properties. One of the issues limiting the use of Li metal is the formation and instability of a solid electrolyte interphase (SEI). Therefore, understanding the exact structure and structural dynamics of the SEI in new battery designs is valuable. Here, we observe the SEI in a new electrolyte.
Read the full experiment brief
Visit the K3 IS product page to find other examples of low-dose imaging via real-time electron counting.
Application: Dynamic in-situ lithiation of NiS-filled carbon nanotubes
Minimizing beam-induced effects is key for accurately studying lithiation phenomena during in-situ experiments. Researchers used low-dose techniques alongside the K3® IS direct detection camera to capture video data of the dynamic lithiation front progress through a carbon nanotube filled with NiS. Dose rates of just 2.1 e-/Å2/s were used to capture high-quality video data.
Read the full experiment brief
Visit the K3 IS product page to find other examples of low-dose imaging via real-time electron counting.
Application: Observing beam-induced dendritic growth over two different timescales
Imaging rapidly changing specimen morphology over a large field of view is a frequent experimental requirement. Observing dendritic growth in Li metal anodes can be necessary to develop strategies for preventing this unwanted behavior. The example below highlights low-dose observations of beam-induced dendritic growth on Cu dendrites. Various image and video analysis techniques were then used to segment the images acquired with the K3 IS camera and then quantify growth rates.
Read the full experiment brief
Application: Observing the effects of oxygen activity on NCA battery electrodes via in-situ EELS
Advances in detector speed and sensitivity, and data processing techniques, also allow for in-situ analysis of elemental composition and chemical state. As an example, microscopists heated a LiNi0.8Co0.15Al0.05O2 (NCA) electrode specimen to understand better its temperature stability and how any changes could affect its performance or safety.
Read the full experiment brief
Visit the Gatan YouTube channel for detailed tutorials on using the in-situ data handling tools available in the Gatan Microscopy Suite® (GMS) software.
The success of any microscopy experiment begins with proper specimen preparation. The best technique for the material depends on the intended analysis (e.g., TEM vs. SEM analysis), specimen morphology (e.g., powder material vs. assembled coin cell), material sensitivity, among other factors. Critical to the specimen preparation process is the ability to cut, polish, etch the specimen without introducing artifacts that obscure the actual state and morphology of the specimen of interest. Gatan specializes in broad ion beam tools for preparing SEM and TEM specimens rapidly and with minimal artifacts from the preparation process.
SEM preparation
Surface analysis of an electrode or similar material can be straightforward and may only require deposition of a thin conductive layer (such as amorphous carbon or a thin metal layer) to avoid charging during observation in the SEM.
Researchers may be interested in viewing the interior structure of their electrode or cell assembly, and in those cases, cross-section preparation is necessary. Gatan’s broad argon ion beam tools can prepare cross-section specimens of larger area than possible with a focused ion beam (FIB) approach and do so through a simpler preparation process with lower cost and faster preparation time.
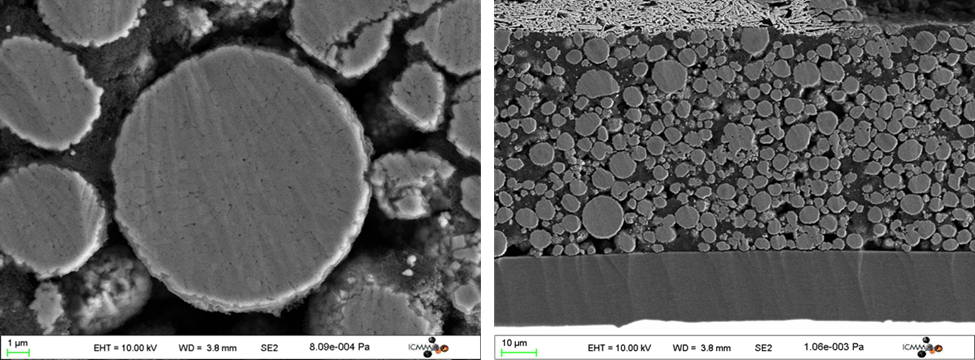
Visit the Ilion® II product page for more information on the tool and application examples.
TEM preparation
If the material of interest is a powder, it is often possible to simply disperse the powder onto a TEM grid with some amorphous support (e.g., C, SiO2) and do imaging on thin areas without any extra preparation. In other cases, it may be necessary to prepare site-specific specimens or cross-sections using a FIB. Utilizing the PIPS™ tool as a final step after FIB can remove surface contamination (such as redeposition of milled material from the FIB) or surface oxidation and leave a clean surface for TEM analysis.
Application: Argon ion polishing of focused ion beam specimens in PIPS II system
Key features of the PIPS II system – including focused ion beams at low energies (~1 mm in diameter), an X,Y alignment stage, an optical camera along with DigitalMicrograph® imaging software, stationary milling, and custom milling angles – make it possible to polish FIB specimens efficiently at <300 eV. Using the PIPS II as the last step in the FIB preparation process improves the specimen quality and removes re-deposited material.
Most battery materials and energy storage materials, in general, are relatively air-sensitive. Certain materials can show extreme air-sensitivity in their pristine state (such as Li metal anodes). Incorporating air-free specimen transfer techniques into the entire experiment workflow is a very important step that is easily overlooked. Furthermore, the stability of the material in the microscope itself can be a challenge, as the electron beam can cause unwanted changes or completely destroy the specimen during analysis. There are several available tools from Gatan to assist with appropriately handling air-sensitive and beam-sensitive specimens.
Air-free transfer of SEM specimens
The ILoad™ system provides a specialized specimen transfer pod and airlock system for Gatan specimen preparation tools and an SEM. A specimen can be loaded inside a glovebox and into the transfer pod, allowing the specimen to be safely transported out of the glovebox while remaining in an inert gas atmosphere with positive pressure to prevent air from entering the chamber. The transfer pod interfaces with airlocks on the PECS™ tool in addition to a customized airlock for an SEM.

Air-free transfer of TEM specimens
The ILoad system additionally interfaces with the PIPS tool, allowing you to bring a specimen out of the glovebox and into the PIPS with the same inert gas atmosphere. After specimen preparation, the specimen can be brought back to the glovebox and easily loaded into the model 648 holder for transfer under vacuum or inert gas atmosphere into the TEM.
Cryo-transfer of TEM specimens
An increasingly popular technique for studying beam-sensitive materials is the use of cryo-transfer holders to freeze the specimen at cryogenic temperatures for observation. Using a cryo-transfer holder minimizes unwanted surface reactions that may happen at room temperature, and also improves specimen stability under the electron beam.
Application: Imaging discrete ions at a liquid-solid interface using low-dose cryo-electron microscopy (cryo-EM) and electron counting
Studying the structure of an electrode-electrolyte interface and its evolution is critical for fully characterizing the electrochemical behavior and performance of a battery system. This is often done using liquid cell techniques. However, recent approaches have also integrated techniques popular with cryo-EM to freeze sensitive specimens as a way of preserving their structure. The example below shows an experiment combining these techniques to look at the liquid-solid interface of a sample Au nanorod—an experiment widely applicable to the large field of energy storage materials.
Read the full experiment brief