What is EDS?
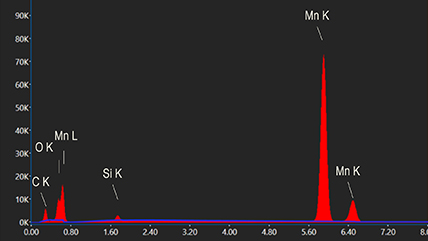
One of the many types of signals generated in a scanning transmission electron microscope (STEM) is characteristic x-rays: incident electron creates a vacancy in an inner orbital of the atom, then an electron from a higher orbital fills this vacancy and emits x-rays. '
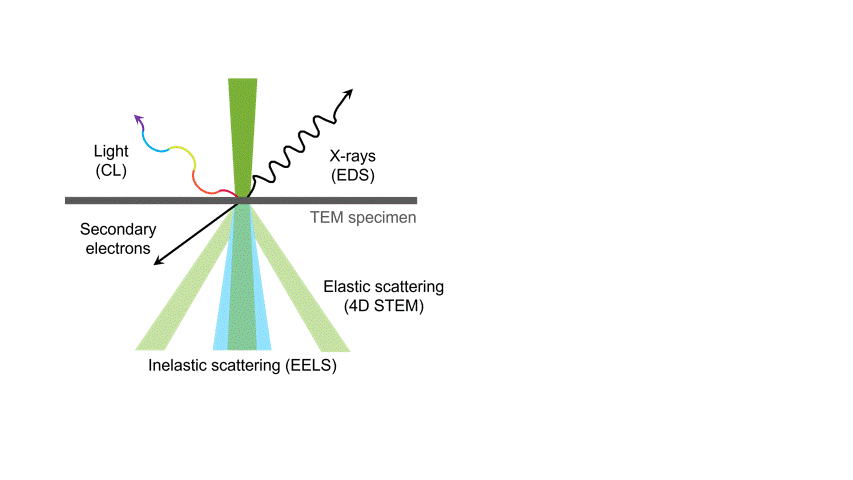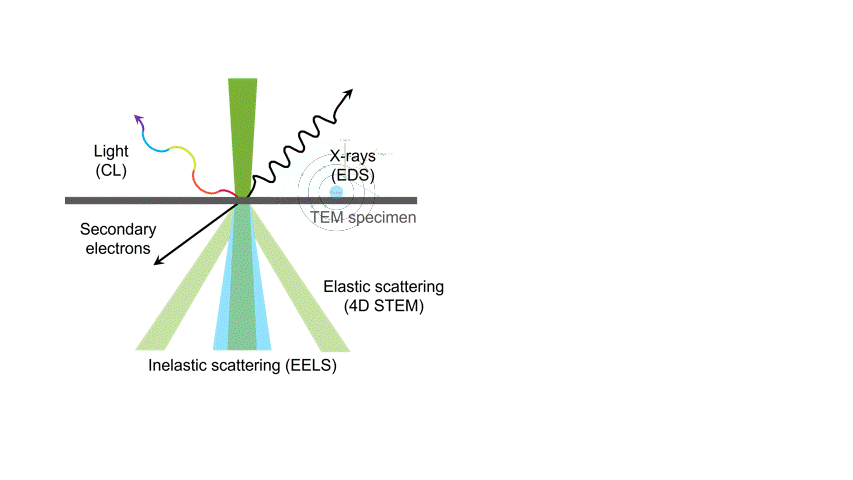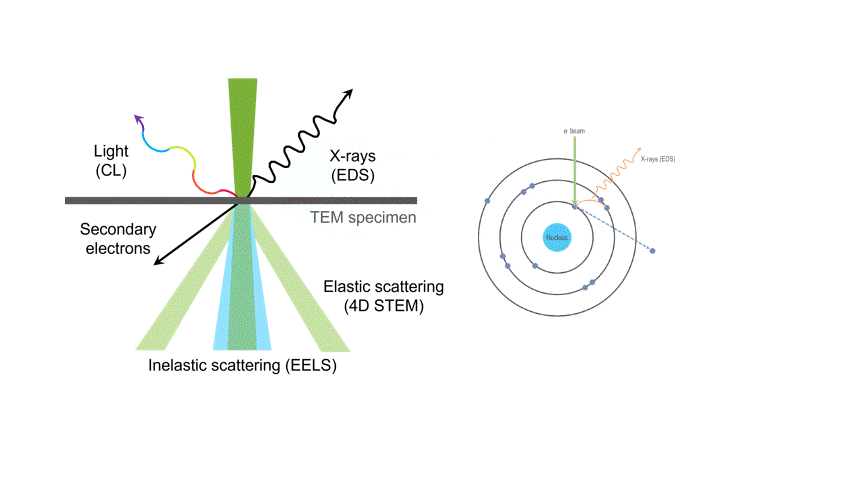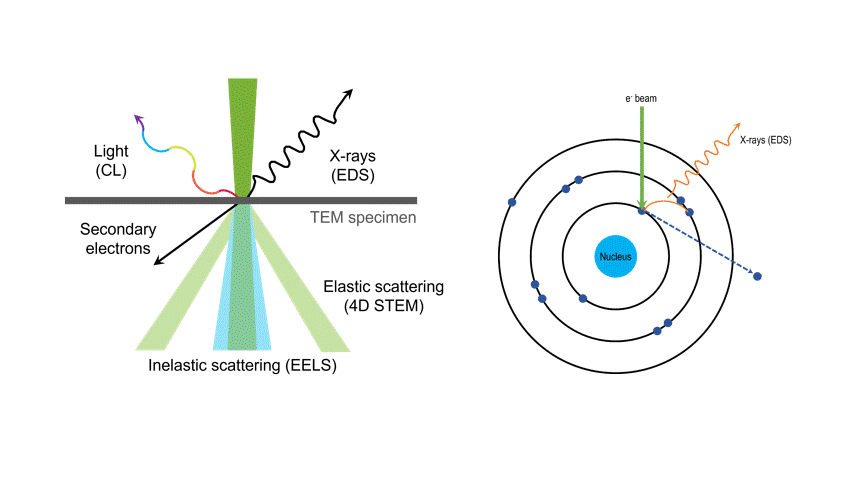
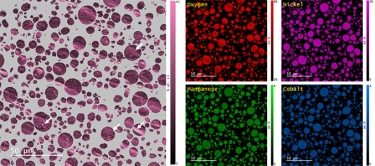
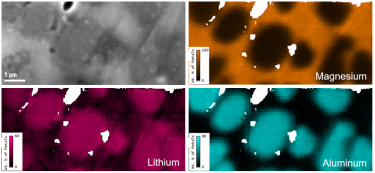
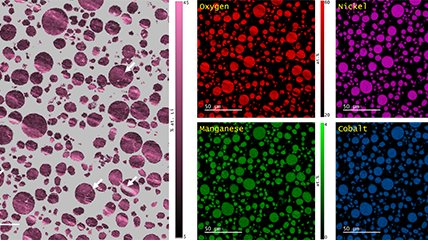
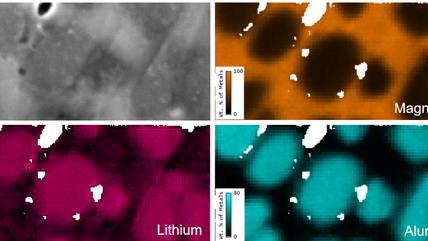
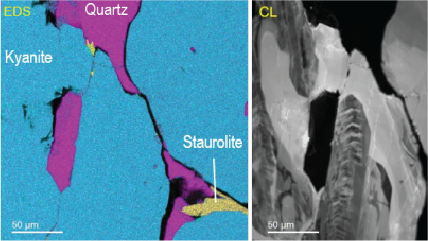
Energy dispersive x-ray spectroscopy (e.g., EDS, EDX, XEDS) is an analytical technique that uses these characteristic x-rays for elemental and compositional analysis of materials. Unlike electron energy loss spectroscopy (EELS), EDS requires minimal setup on the microscope, is used on semi-thin to bulk specimens, and offers a high signal-to-background ratio. This technique is key to understanding foreign materials, coating compositions, small component materials, evaluating corrosion, phase identification, and distribution.
Gatan offers eaSI™ EDS, the most effective and efficient STEM EDS that leverages the EDAX Elite T EDS detector and DigitalMicrograph® software.
Since the changing local chemical environment atomic bonding has little effect on EDS, there are better techniques than this one for specimen chemical analysis. However, it is excellent to determine the elemental distribution of a sample. Combined with electron energy loss spectroscopy (EELS), you can easily analyze your specimen's chemical and compositional makeup.
Unlike similar techniques, EDS requires minimal setup and is used in semi-thin to bulk specimens; thickness options are unlimited. EDS also offers a high signal-to-background ratio (SBR). Some limitations of EDS include non-local fluorescence; low-Z limits; and limited thin-film signal-to-noise ratio (SNR).
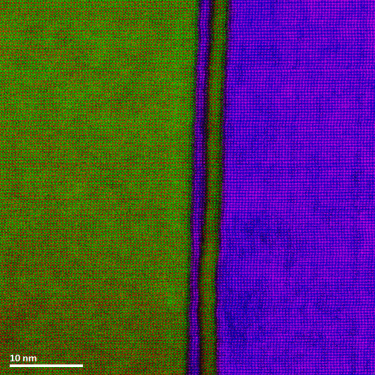
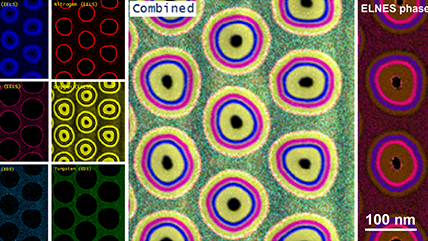
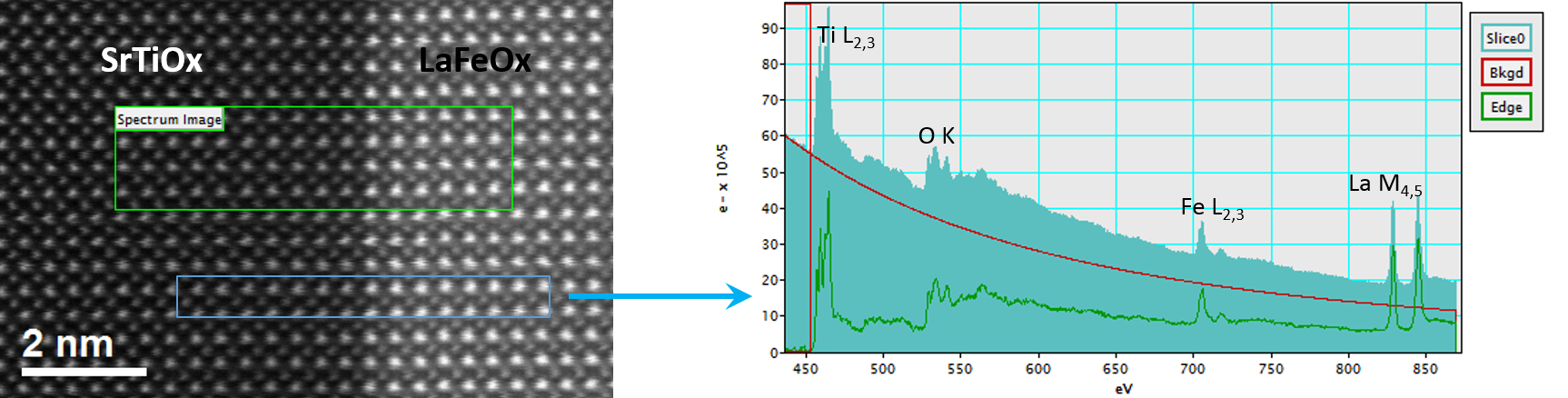
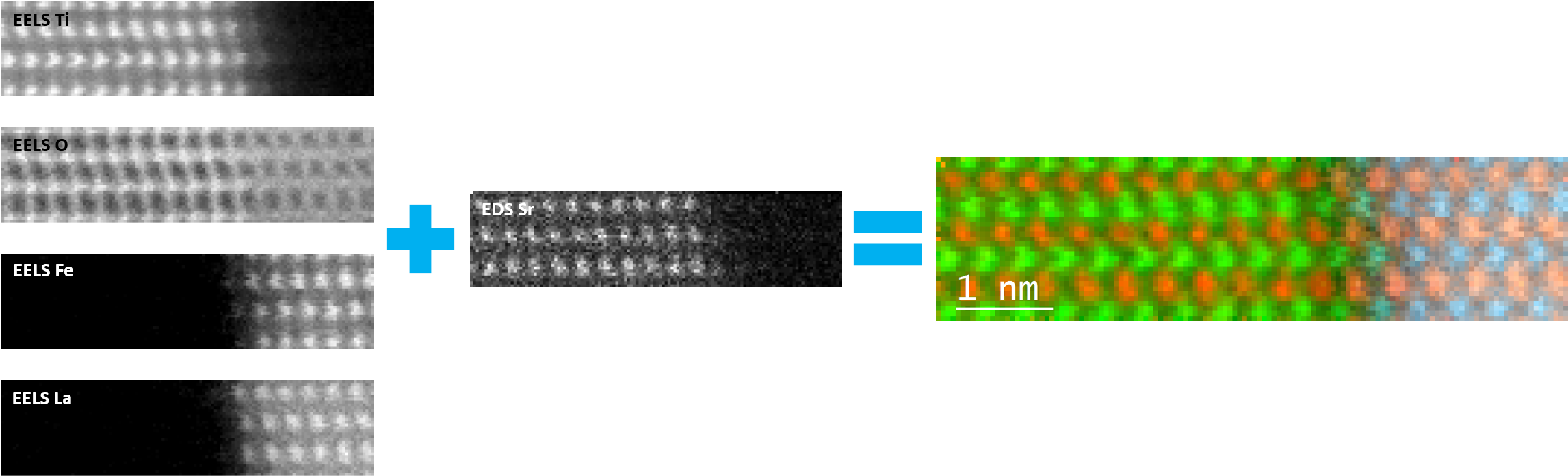
In the example below, the Sr L2,3-edges at 1940 eV were not within the 0 – 850 eV energy range (top) after an EELS analysis was run on a semiconductor sample. When spectrum imaging was used to obtain the EDS Sr elemental map in combination with the EELS Ti, Fe, and La maps (bottom), the collective color map shows the distribution of each element across an extended energy range.
For more information about comparing this technique to other analytical techniques, please visit EELS.info, an educational site.