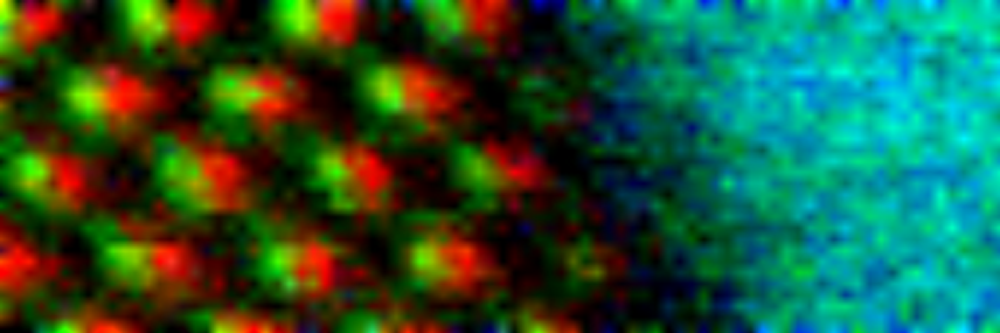
Atomic Resolved EELS color map of GaAs/Ga2O3
Paolo Longo, Ph.D., Gatan, Inc.
Sample courtesy of University of Glasgow
Microscope courtesy of Dr. Yan Xin at Florida State University, Tallahassee, FL
Acknowledgement to Dr. Toshiro Aoki at Jeol USA (now at ASU) for helping set up microscope for experiment.
Dumbbell structure of GaAs on left hand side of color map is clearly resolved, and Ga and As atomic columns are visible. Interface region shows presence of Ga2O monolayer responsible for keeping Fermi level unpinned, which allows the electronic device to be turned on or off.
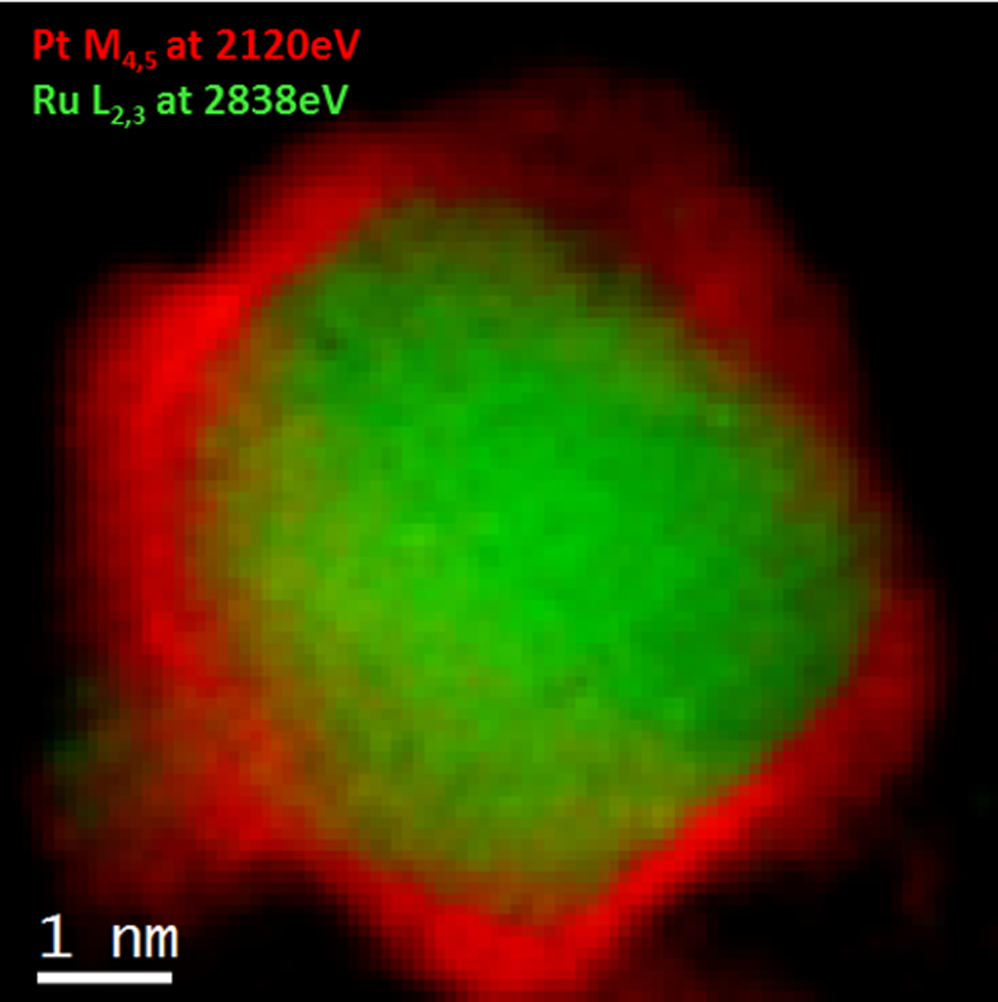
Atomic level EELS color map of a Pt/Ru catalyst nanoparticle
Paolo Longo, Ph.D., Gatan, Inc.
Sample courtesy of Professor Gianluigi Botton at CCEM, Hamilton, ON, Canada
Microscope courtesy of CCEM, Hamilton ON, Canada
Acknowledgement to Dr. Andreas Korinek at CCEM for helping set up microscope for experiment.
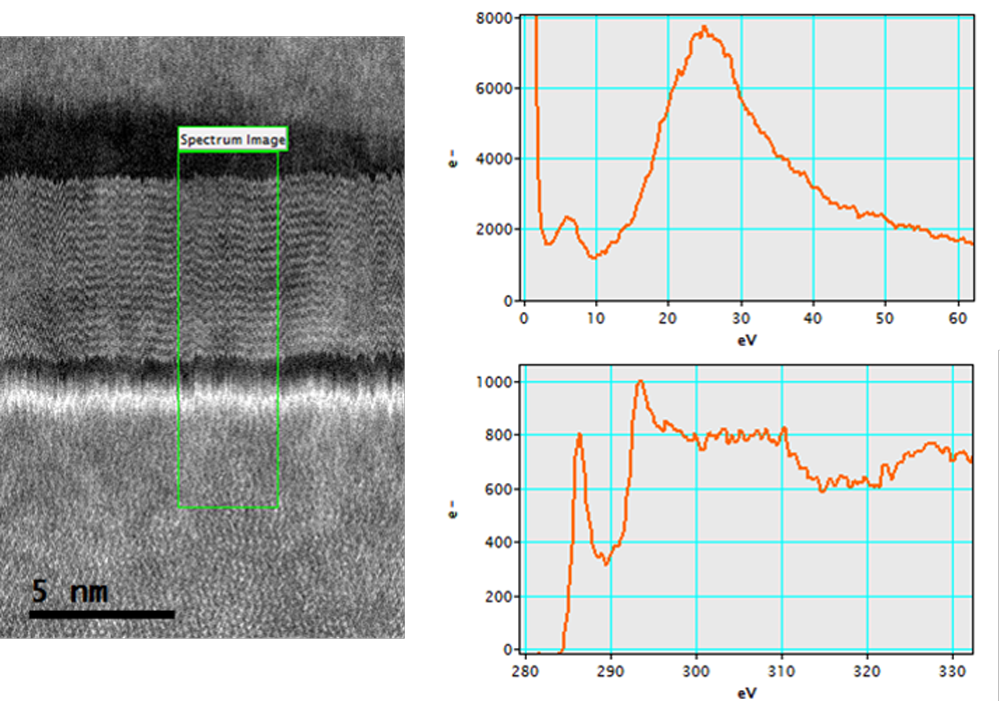
Fast atomic DualEELS analysis at 60 kV of graphene layers after graphitization process of SiC
Paolo Longo, Ph.D., Gatan, Inc.
Sample courtesy of Dr. Giuseppe Nicotra at IMM-CNR, Catania, Italy
Microscope courtesy of IMM-CNR, Catania, Italy
Methods
Probe-corrected ARM 200F TEM/STEM microscope; C-FEG emission gun; GIF Quantum® ER system; voltage: 60 kV; STEM mode; EELS low-loss spectrum (0 – 500 eV) exposure time: 0.01 ms; EELS core-loss spectrum (70 – 570 eV) exposure time: 10 ms; total exposure time: <2 min
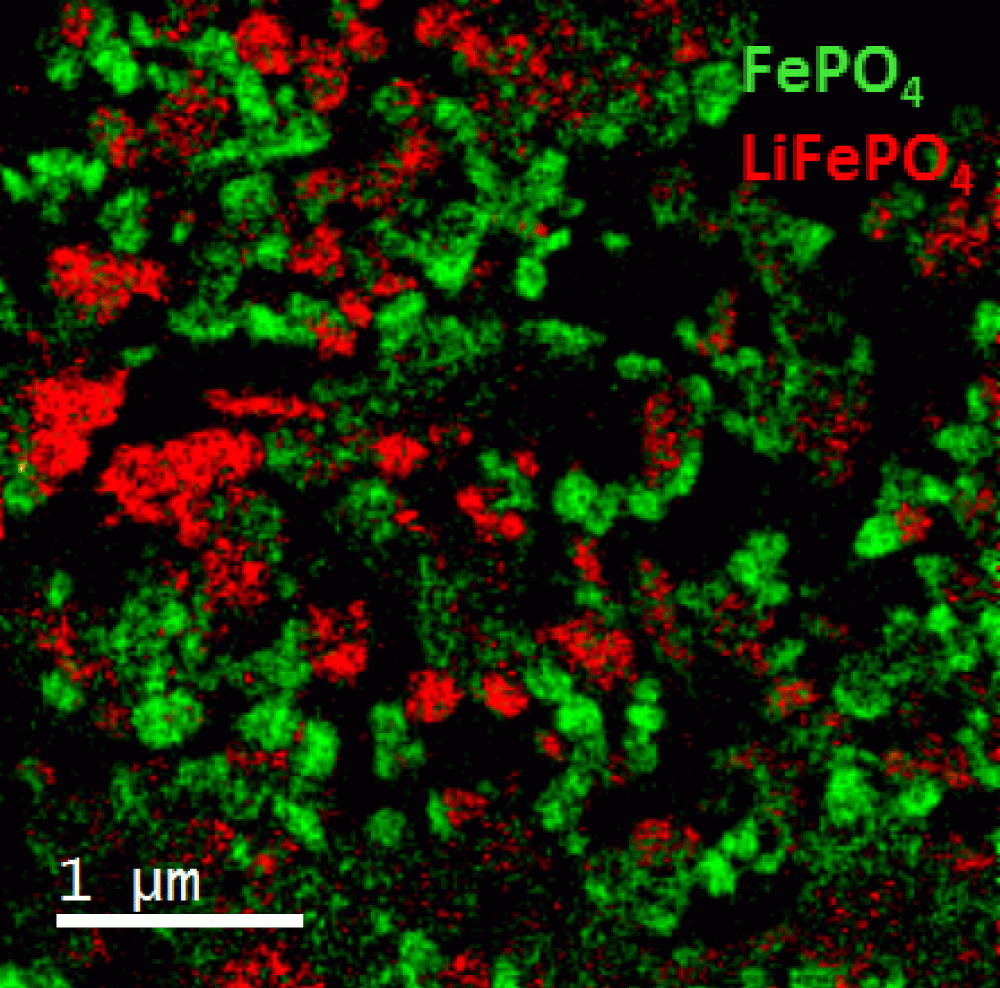
EELS color map showing the distribution LiFePO4 (red) and FePO4 (green) particles from a battery electrode charged to half cycle
Paolo Longo, Ph.D., Gatan Inc.
Sample courtesy of Dr. Joshua Sugar at Sandia National Lab, Livermore, CA
Methods
FEI F20 TEM/STEM microscope; S-FEG emission gun; GIF Quantum® ER system; voltage: 200 kV; EFTEM SI mode from 680 – 730 eV; slit width: 2 eV ; step size: 1 eV; total exposure time: <4 min
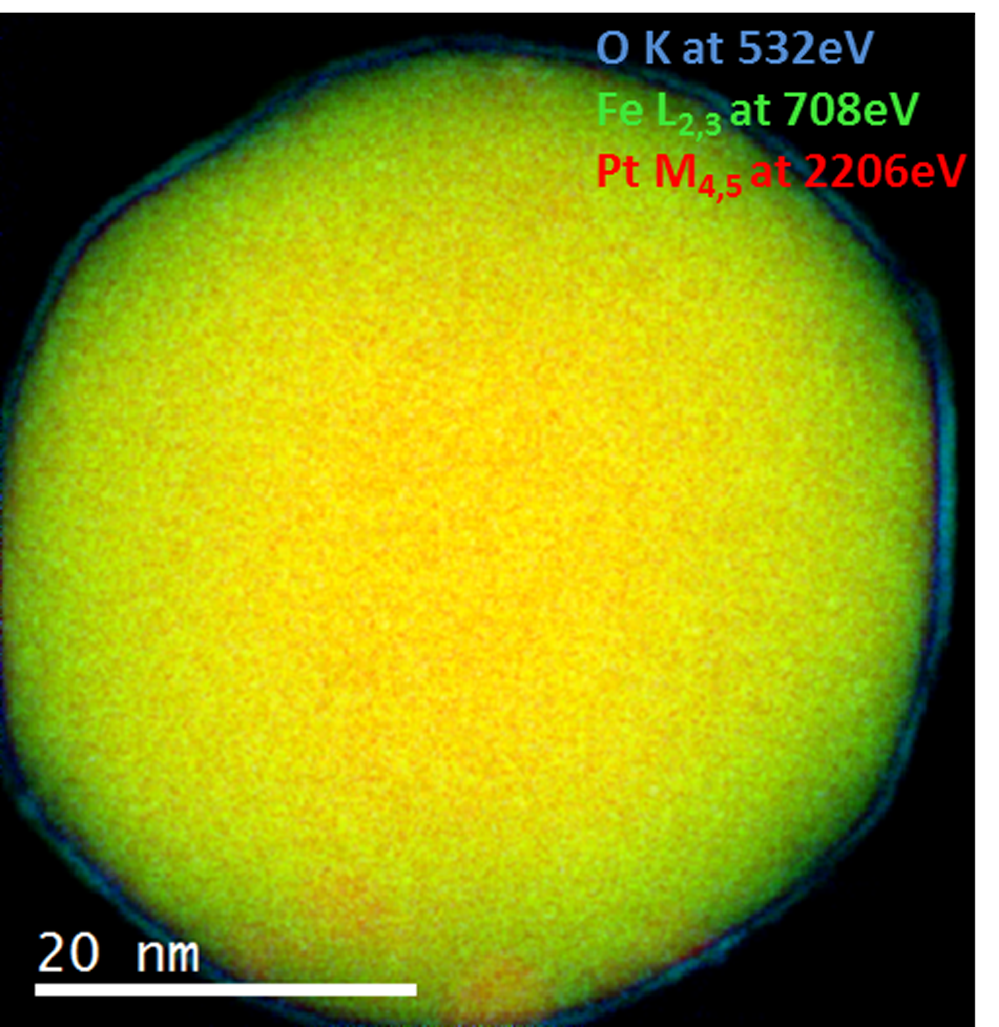
EELS color map of a Pt/Fe catalyst nanoparticle
Paolo Longo, Ph.D., Gatan, Inc.
Sample courtesy of Professor Gianluigi Botton at CCEM, Hamilton, ON, Canada
Microscope courtesy of CCEM, Hamilton, ON, Canada
Acknowledgement to Dr. M. Bugnet at CCEM for helping set up microscope for experiment.
Pt/Fe nanocatalysts are among the most promising candidates for accelerating oxygen reduction reaction occurring at the cathode in a proton exchange membrane fuel cell.
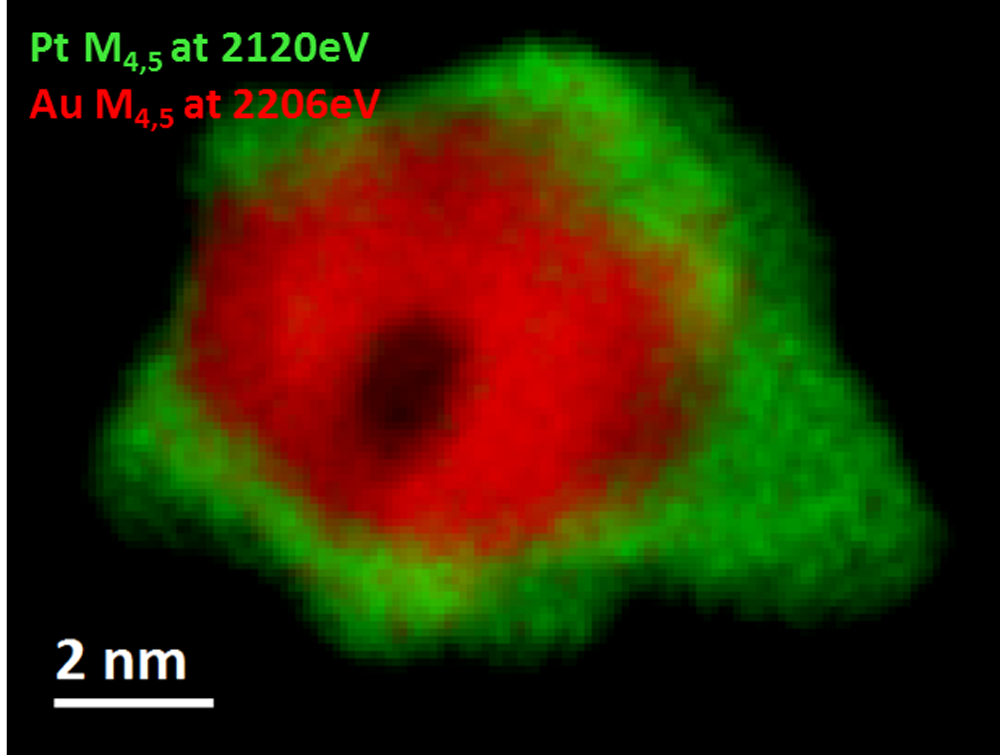
EELS color map of a Pt/Au catalyst nanoparticle
Paolo Longo, Ph.D., Gatan, Inc.
Sample courtesy of Professor Gianluigi Botton at CCEM, Hamilton, ON, Canada
Microscope courtesy of CCEM, Hamilton, ON, Canada
Acknowledgement to Dr. Andreas Korinek at CCEM for helping set up microscope for experiment.
EELS data taken using a probe and image-corrected FEI Titan TEM/STEM microscope equipped with X-FEG emission gun and a fully loaded GIF Quantum® ERS system. Au M4,5-edges at 2206 eV (red) and Pt M4,5-edges at 2206 eV (red).
Methods
Voltage: 200 kV; data taken in STEM mode; EELS core-loss spectrum (1400 – 3400 eV) exposure time: 20 ms; convergence angle: 19 mrad; collection angle: 34 mrad; beam current: 180 pA; total exposure time: <5 min
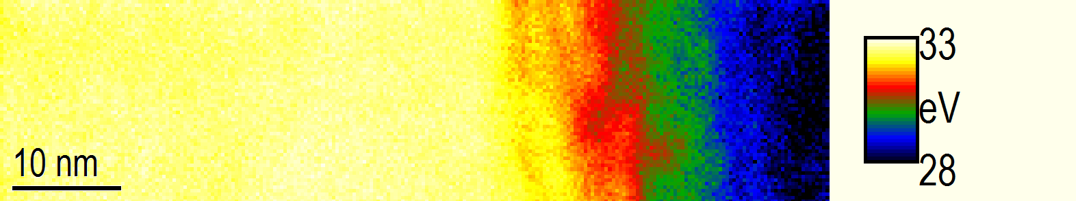
Plasmon peak position map going from diamond (yellow) to amorphous carbon (blue)
Paolo Longo, Ph.D., Gatan, Inc.
Sample courtesy of Dr. Jaco Oliver at NMMU, Port Elizabeth, South Africa
Microscope courtesy of NMMU, Port Elizabeth, South Africa
Analysis of the changes in local carbon structure caused by self-implantation of carbon into diamond and chemistry.
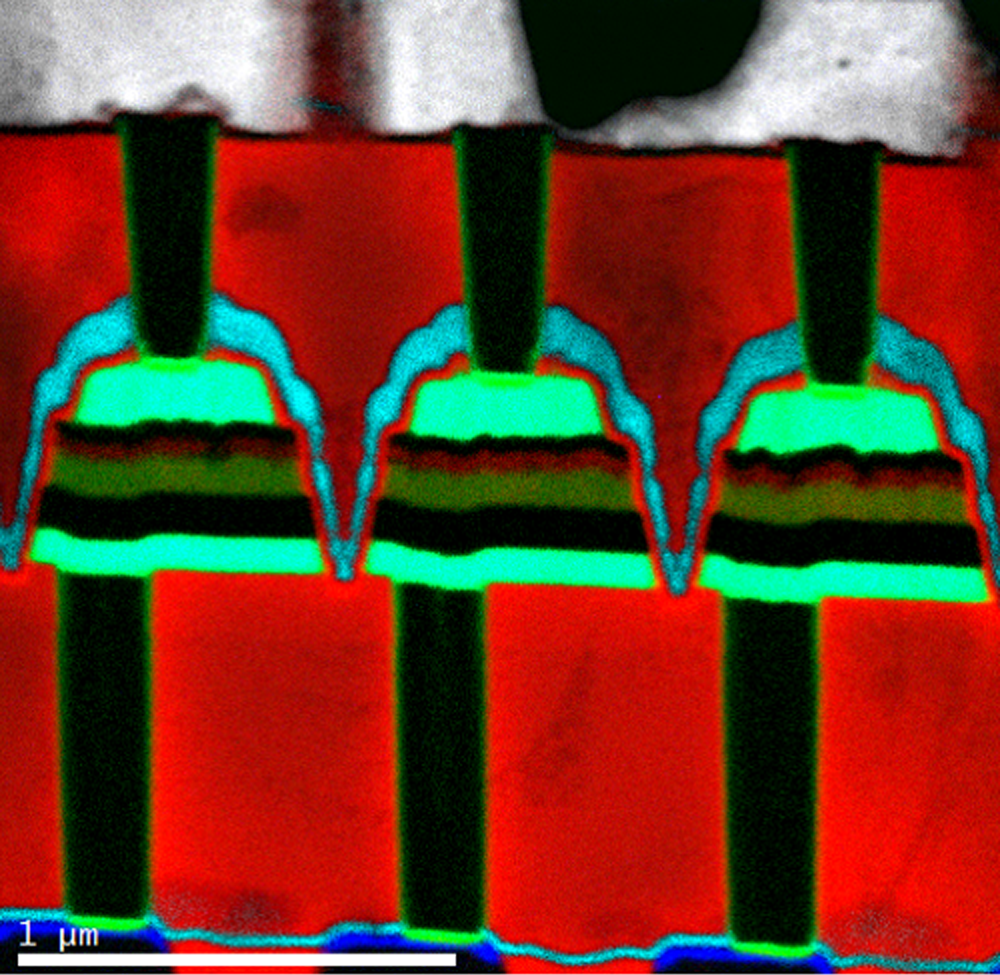
EELS color map of a magnetic device
Paolo Longo, Ph.D., Gatan, Inc.
Legend
Cu L at 931 eV (grey); O K at 532 eV (red); N K at 401 eV (light blue); Ti L at 456 eV (green); Co L at 779 eV (blue)
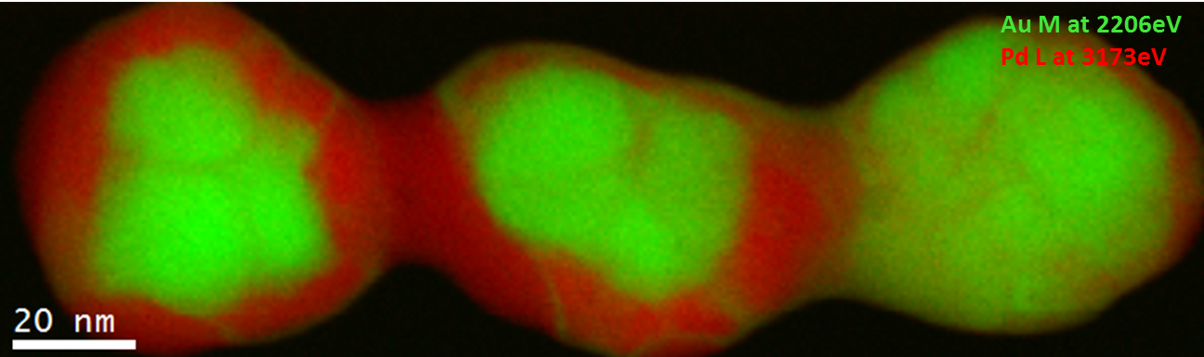
EELS color map of a Pd/Au catalyst particle
Paolo Longo, Ph.D., Gatan, Inc.
Sample courtesy of Professor Jianfang’s group, Chinese University, Hong Kong
Microscope courtesy of IBM, Fishkill, NY
Pd/Au alloys have attracted a lot of interest due to their resistance at high temperatures, and this explains their use in several fields, such as CO and hydrocarbon oxidation, synthesis of vinyl acetate monomer, hydrocarbon hydrogenation, and many others. Pd is the catalytic center, whereas the Au has the effect of changing the chemical properties at the surface of the Pd-Au alloy. This change influences the catalytic properties. Hence, the study of the chemistry and the elemental distribution is important to understand the properties of the whole catalyst system.
Pages