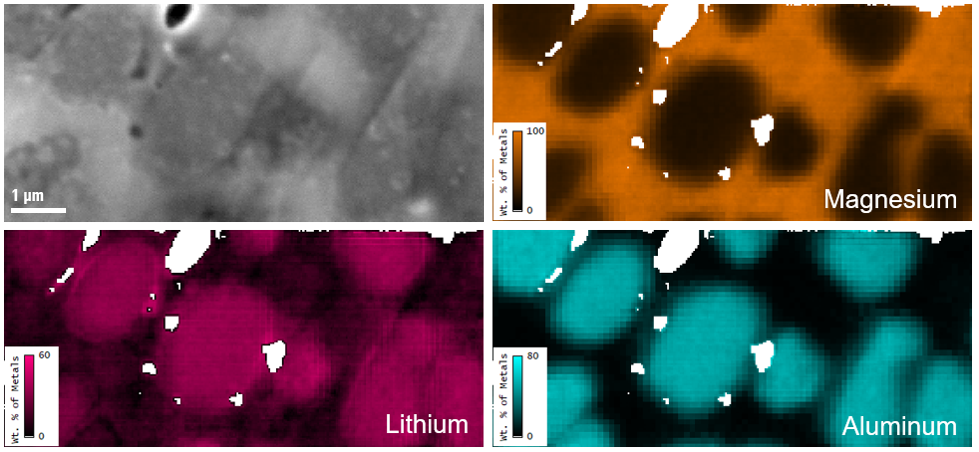
Quantitative mapping of lithium in the scanning electron microscope
Secondary electron image and elemental metal fraction maps (by wt. %) of the same region of the MgLiAl alloy; white pixels are regions excluded from the analysis due to influence of topography.
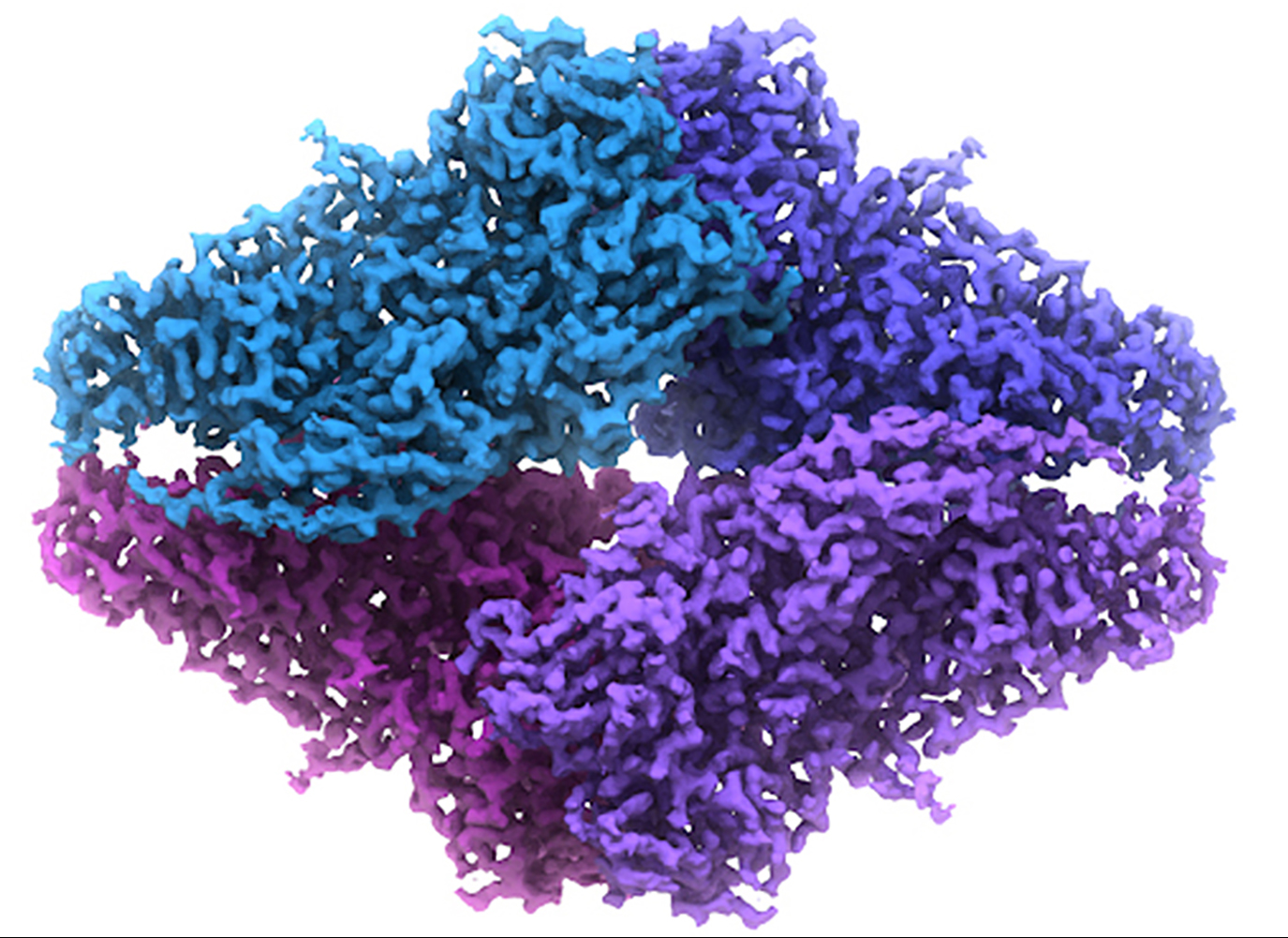
Scientists reach 2.2 Å using cryo-electron microscopy
Bartesaghi et al., Science 348 (6239): 1147-1151
2.2 Å resolution single-particle reconstruction of β-galactosidase enabled by GIF Quantum LS imaging filter with K2 Summit camera.
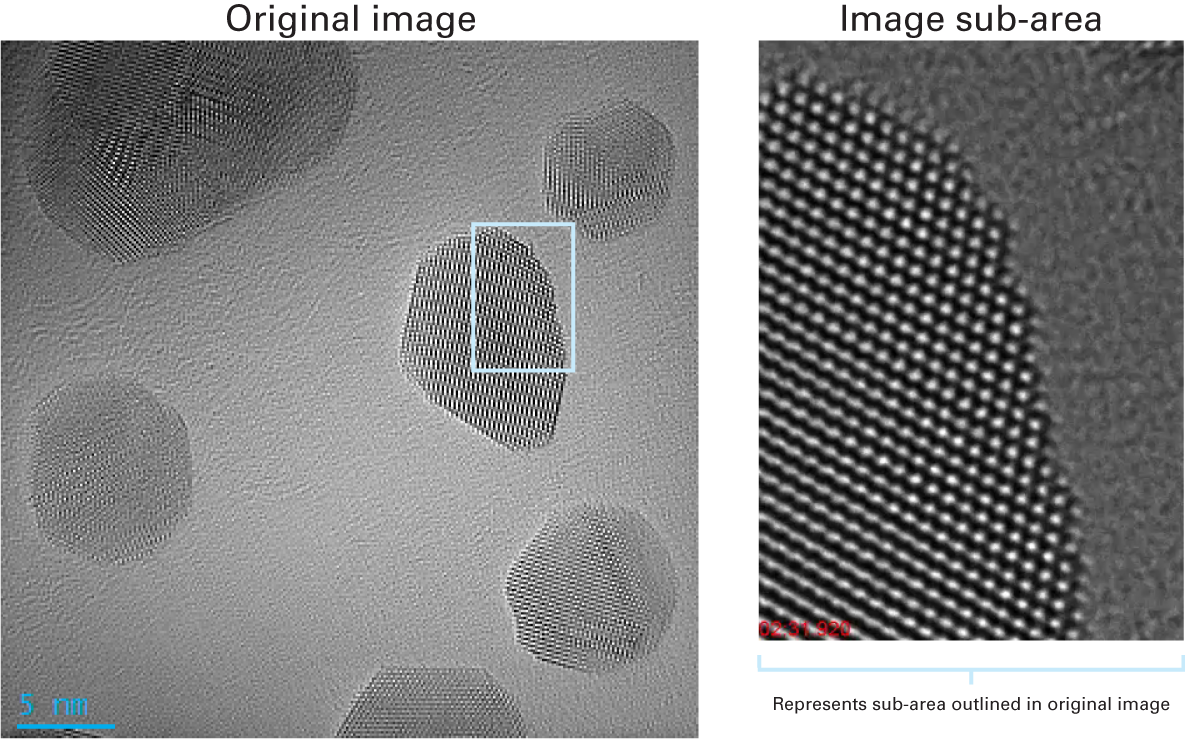
Au nanocrystal reorientation
Video courtesy JEOL Japan and Gatan, Inc., US
A single full field of view image (left) shows the high sensitivity and high signal-to-noise in a 40 ms frame. The right image is a single frame from a video that showcases step motion on the surface of a Au nanocrystal under the electron beam.
Sample: gold (Au) nanoparticles; beam energy: 300 kV; original image size: 4k x 4k; frame rate: 25 fps
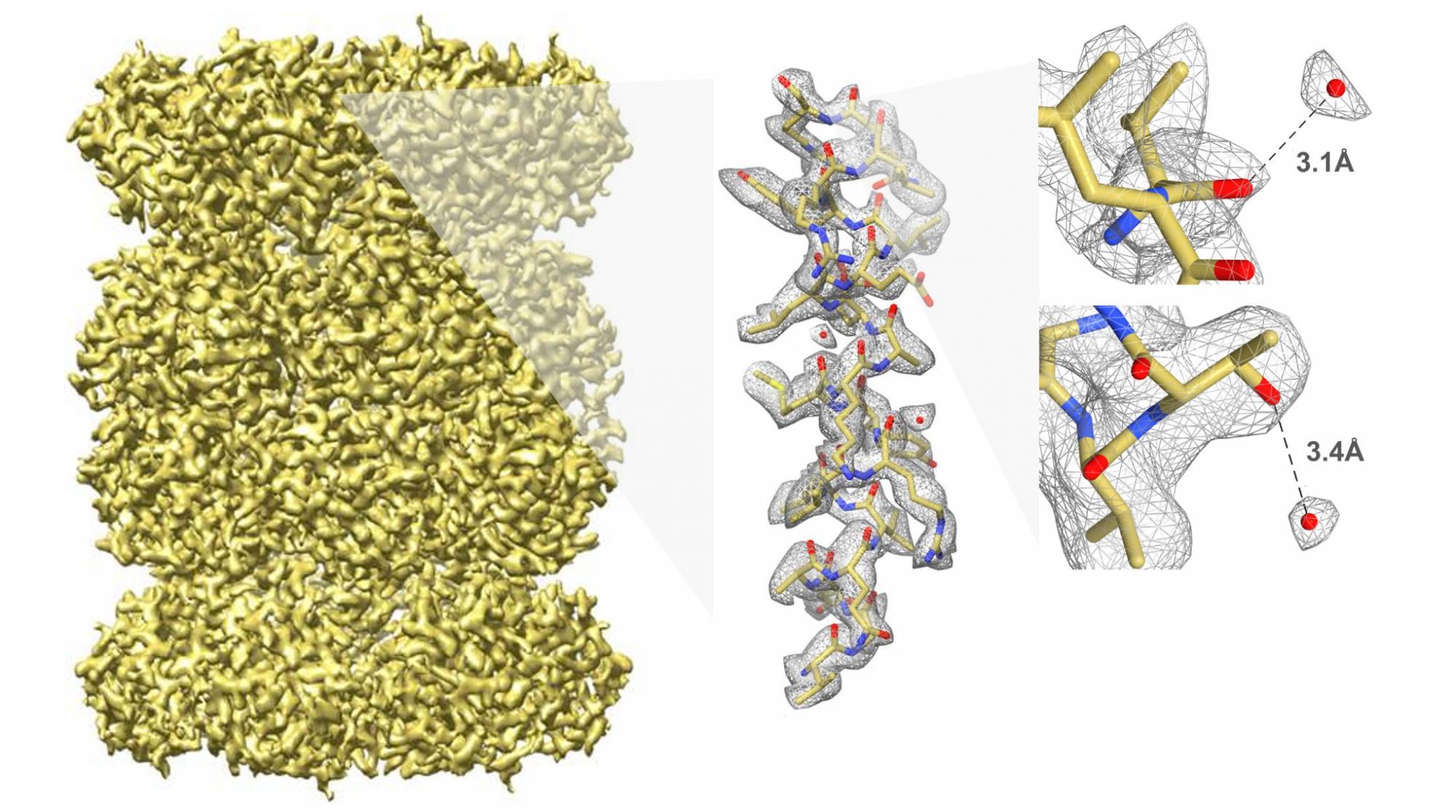
Breaking the 3 Å barrier
Image courtesy of Melody Campbell and the National Resource for Automated Molecular Microscopy, US.
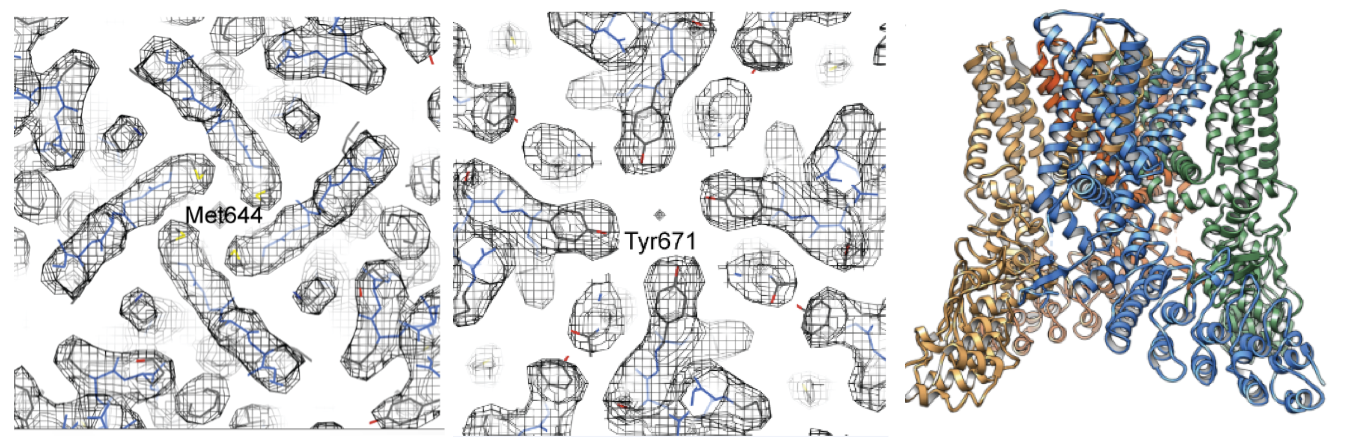
The above series of images show a 3D reconstruction of the 20S proteasome at 2.8 Å resolution. On the right, the oxygen atom (red) found in a single water molecule is separated from the surrounding structure by 3.1 and 3.4 Å, respectively. Studying this molecule at high resolution demonstrates that scientists can identify water molecules and hydrogen bonding in structures solved by cryo-EM using the K2 Summit camera. eLife 2015;4:e06380
First ~700 kDa protein structure with D7 symmetry identified at 3.3 Å resolution using cryo-EM
Li, X.; Mooney, P.; Zheng, S.; Booth, C. R.; Braunfeld, M. B.; Gubbens, S.; Agard, D. A.; Cheng, Y.
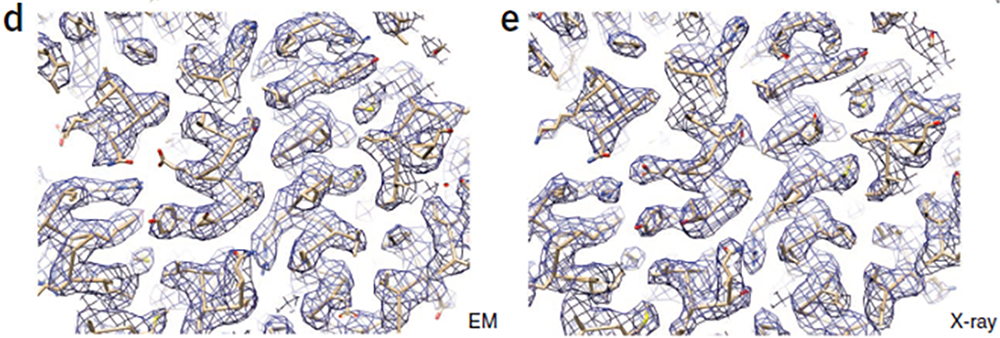
Using the K2 Summit® camera, single-particle cryo-EM was able to match the x-ray crystallography result. (d) Portion of the cryo-EM density map showing clear side-chain densities. The docked atomic structure was refined to fit the density map by a molecular dynamic flexible fitting procedure.
First 3.4 Å TRPV1 structure solved by cryo-EM
Liao, M.; Cao, E.; Julius, D.; Cheng, Y.
K2 Summit camera was used to determine the structure of a mammalian TRP channel, TRPV1, at 3.4 Å resolution, breaking the side-chain resolution barrier for membrane proteins without crystallization. Nature 504, 107–112, 2013
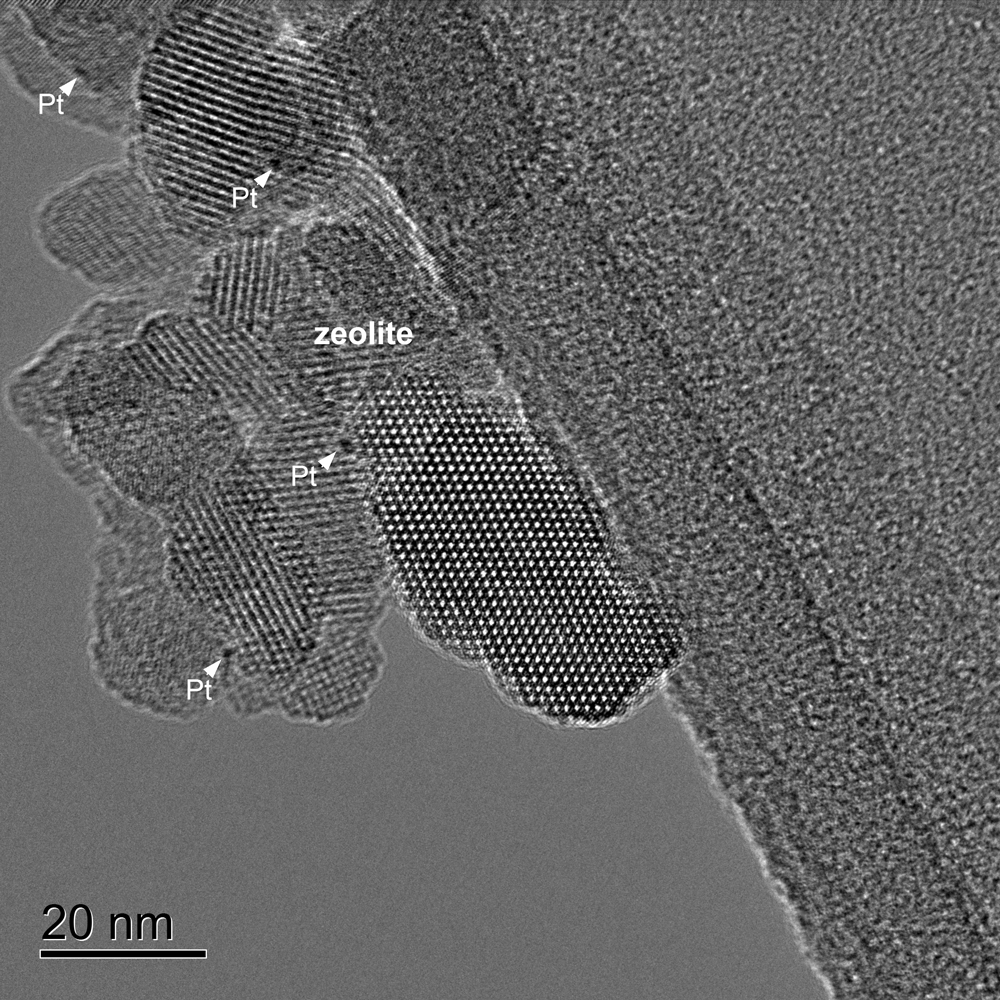
High resolution image of a zeolite sample containing small metal Pt particles
Chevron zeolite SSZ-57
Methods
Image was captured with OneView® camera
TEM magnification: 255kx
Electron energy: 200 keV
Exposure time: 2 s
Drift correction: On
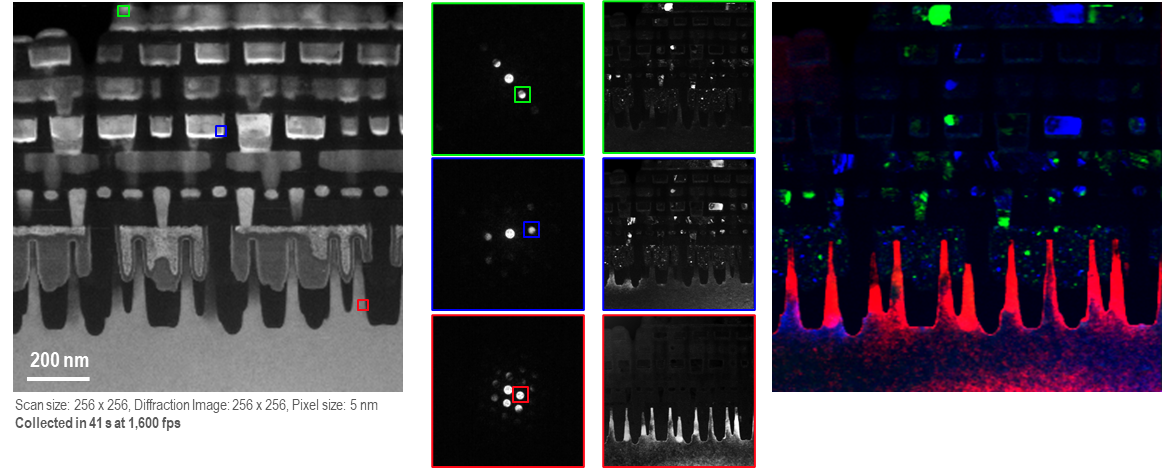
4D STEM and Virtual Aperture Imaging with ClearView
The ClearView camera can acquire 4D STEM datasets at up to 1,600 fps, enabling high-throughput and large area 4D STEM acquisition. Using built-in tools in DigitalMicrograph, users can probe the diffraction image at different locations within the 4D STEM spectrum image and reconstruct RGB images from different virtual dark-field images.
Images acquired at 200 kV using the ClearView camera and STEMx system (eaSI).
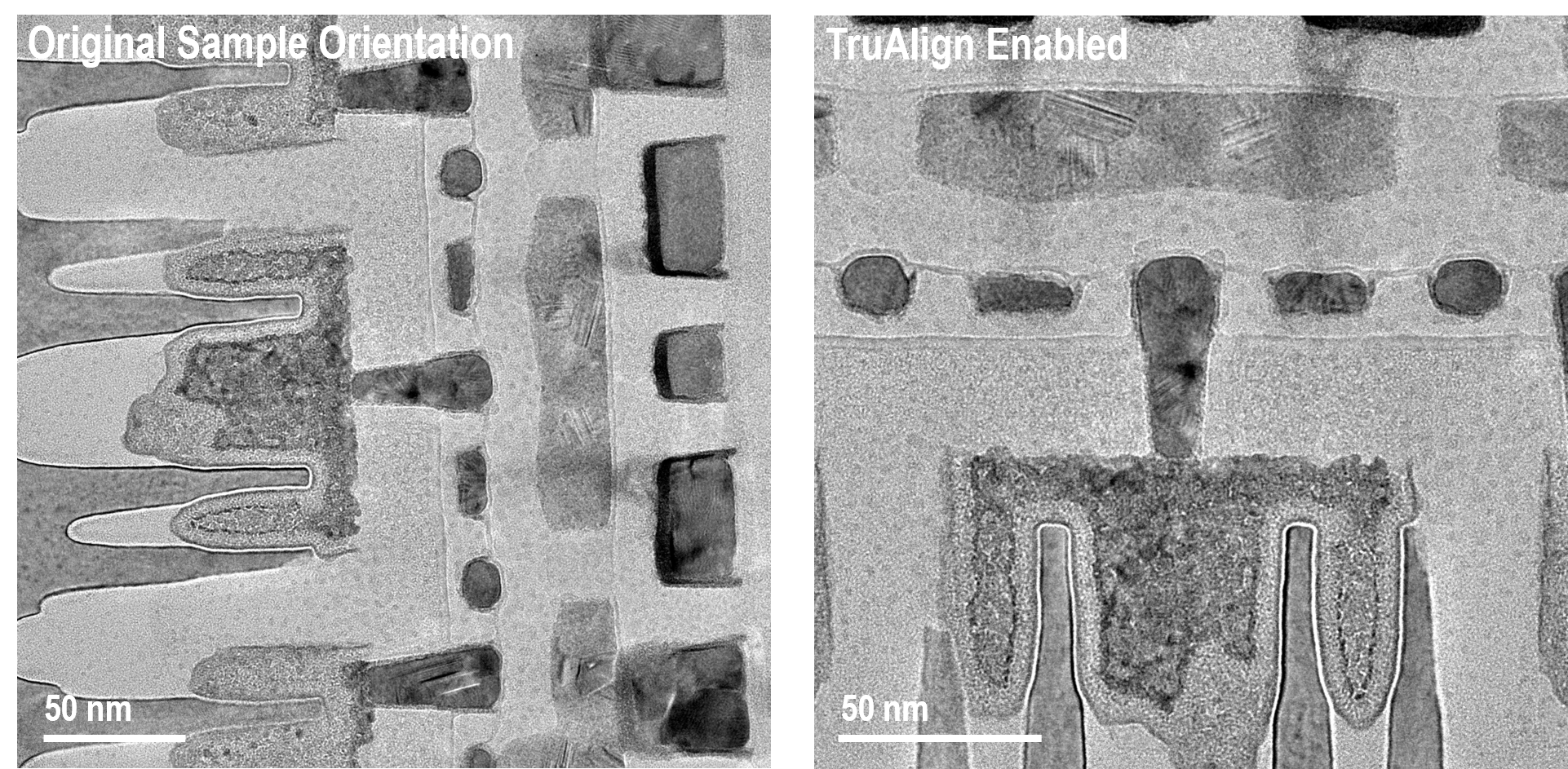
Image samples at the desired orientation with TruAlign
TruAlign enables a digital rotation of the ClearView camera image so that data can be acquired at the desired sample orientation.
Images acquired with a ClearView camera at 200 kV and . The original sample orientation image was acquired at 4k x 4k resolution and the TruAlign image was acquired at 2850 x 2850 resolution.
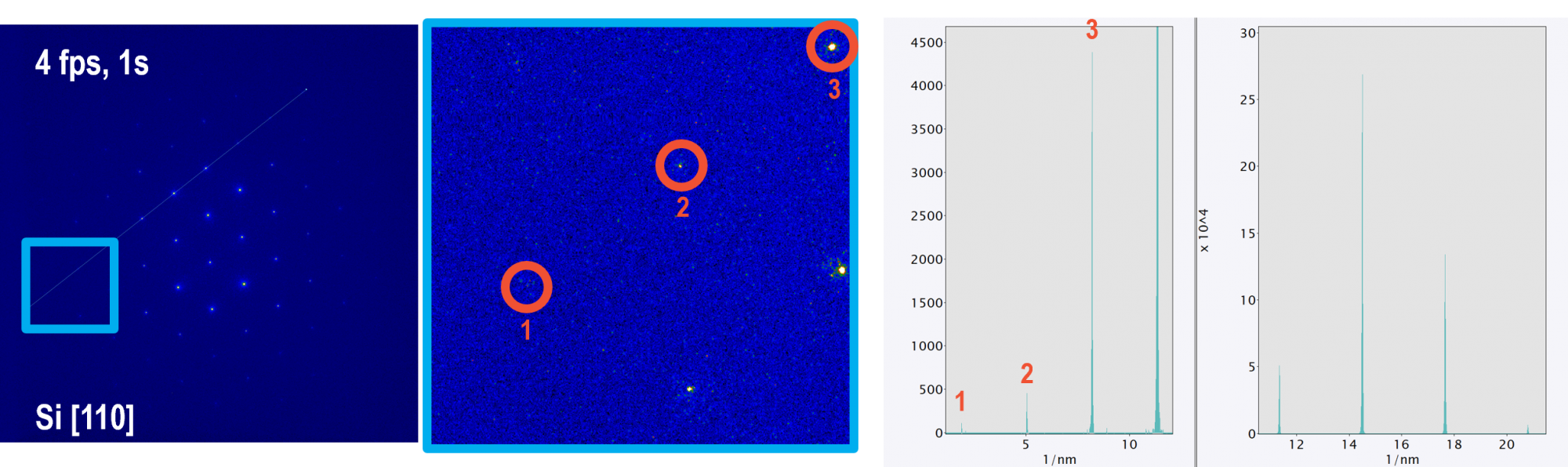
Better resolve faint, high-resolution diffraction spots with ClearView Frame Control mode
Enabling Frame Control mode allows users to better leverage the full dynamic range of the camera and maximize the signal-to-noise in acquired images by reducing the camera framerate and minimizing the total readout noise in an image. The left image shows a selected area electron diffraction pattern of a Si [110] sample. Frame Control mode was used to optimize the signal-to-noise. The diffraction pattern was a 1s acquisition at 4 fps and 4k x 4k resolution. The middle image shows an inset area of the diffraction pattern, showing faint diffraction spots. Spot #1 in particular is very faint.
Pages