Imaging extremely beam-sensitive materials with Metro
Introduction
Collecting high-quality transmission electron microscopy (TEM) data from extremely beam-sensitive materials can be exceedingly challenging. Without proper care, the electron beam will destroy the sample material before adequate data can be collected. An informed experimental approach must use a low electron beam dose condition that balances the amount of irradiation the sample receives from the electron beam with the desired signal-to-noise ratio for the collected data. This collected data can include standard imaging, diffraction, energy dispersive x-ray spectroscopy (EDS), and electron energy loss spectroscopy (EELS), among others.
The sensitivity of the detector collecting the data plays a critical role in studying beam-sensitive materials in the TEM. For imaging and diffraction applications, the most common type of equipment used is a scintillator-based camera like the Gatan ClearView®, OneView®, Rio®, UltraScan®, or Orius®. These cameras deliver excellent imaging capabilities but lack the sensitivity to acquire high-quality data under very low electron beam dose conditions. Direct detection electron counting cameras like the Metro® and K3® cameras are significantly more sensitive than scintillator-based cameras and can collect high-quality imaging and diffraction data under extremely low beam dose conditions (see our Imaging and Improving DQE with Counting and Super-Resolution pages for more detail).
This application note provides an example of using the Metro direct detection electron counting camera to collect data with extremely low beam dose rates. The sample analyzed was a beam-sensitive 2D polymer material, which can handle less than 100 e-/Å2 total dose at 300 kV (85 e-/Å2 total dose at 200 kV) before beginning to degrade significantly. An extremely low dose condition was used in the TEM to search for the sample area of interest, tilt the sample to the desired zone axis, and collect high-quality diffraction and real-space image data within the 85 e-/Å2 total dose threshold. The Metro camera proved sensitive enough to collect all required data under these extremely low-dose conditions.
Materials and Methods
2D-PI-BPDA, the material studied here, is a 2D polyimine thin film material that is part of the larger family of 2D polymers. Characterizing the structure of 2D-PI-BPDA is critical for understanding the structure-property relationships important to its ability to transport ions, adsorb gas, etc. The structure of 2D-PI-BDPA was recently studied in the TEM [1] and shown to have a 4-fold symmetry in the [001] direction with a 30 Å lattice parameter (Figure 1).
![Figure 1: The unit cell of 2D-PI-BPDA from [1].](/sites/default/files/Applications/AN_MetroLowkV/Figure1.png) Prof. Dr. Ute Kaiser and Dr. Haoyuan Qi from Ulm University provided the TEM samples. The 2D-PI-BPDA specimen was prepared by a surfactant-monolayer-assisted interfacial synthesis (SMAIS) method, where a polymer film is formed on a water surface [2]. This film can be directly transferred to a TEM grid by scooping the film up with the TEM grid.
Prof. Dr. Ute Kaiser and Dr. Haoyuan Qi from Ulm University provided the TEM samples. The 2D-PI-BPDA specimen was prepared by a surfactant-monolayer-assisted interfacial synthesis (SMAIS) method, where a polymer film is formed on a water surface [2]. This film can be directly transferred to a TEM grid by scooping the film up with the TEM grid.
A JEOL F200 TEM was used for data collection at 200 keV. The 40 µm condenser aperture was used for imaging and diffraction, and a 10 µm selected area electron diffraction (SAED) aperture was used to collect diffraction patterns. The total beam current was ~0.45 nA.
The 2D-PI-BPDA sample needed to be imaged in the [001] zone axis to view its structure properly. Although the growth and deposition direction typically put the sample close to the zone axis, tilting the sample was still required. The procedure below was followed to minimize the total dose on the sample during data collection.
- Start the Metro camera live view on a vacuum region of the TEM grid to avoid unnecessary sample irradiation.
- Insert the 10 µm SAED aperture and center the aperture within the camera’s field of view.
- Spread the beam to the microscope limit to make the beam as weak as possible.
- At this point, the dose rate measured with the Metro camera was 0.03 e-/Å2/s.
- Switch the TEM to diffraction mode. Switch the Metro camera to diffraction mode. Center the diffraction pattern on the camera using the projector lens alignment.
- Note: If the beam current is low, the Metro camera can collect diffraction data without requiring a beam stop to block the center diffraction spot. See the following application note for more information.
- Move the stage position to search for sample areas with a diffraction pattern indicating a close alignment to the desired [001] zone axis.
- Under these conditions, the Metro camera is sensitive enough to allow the user to see the center diffraction spot and a few low-order diffraction spots in the camera View mode. However, some higher-order diffraction spots can be hard to see to tilt the sample to the zone axis.
- To make the higher-order diffraction spots more visible, the Metro View mode was set to a 0.4 s exposure with a frame averaging over ten frames. This increases the signal for live View images and diffraction patterns but reduces the refresh rate of the View image.
- When a good sample region is found, tilt the sample to the desired zone axis and collect a diffraction pattern.
- Typically, a 10 s acquisition was used to capture diffraction patterns at the extremely low dose condition used.
- When the diffraction pattern acquisition finishes, immediately blank the TEM beam using the DigitalMicrograph beam blanker tool.
- Switch the TEM back to imaging mode. Switch the Metro camera to imaging mode. Remove the SAED aperture.
- Set the desired image capture time. Check that live drift correction is enabled.
- Typically, a 5 or 10 s acquisition was used to capture images at the extremely low dose condition used.
- Start the Metro view, un-blank the beam, and immediately capture an image.
- If the defocus is not correct, use a live FFT to guide the defocus adjustment and correct any stigmation. Quickly capture another image.
- Alternatively, condense the beam to increase the dose rate if the signal is too low and quickly capture another image.
- Based on the dose rate and acquisition time used throughout the process, the sample may be significantly damaged after capturing the diffraction pattern and 2 – 3 images.
- The TEM and camera are then reset to collect diffraction patterns (steps 1 – 4), and the process (steps 5 – 12) is repeated for another sample area.
Results and Discussion
TEM and diffraction data
High-resolution TEM (HRTEM) imaging and diffraction data were collected from multiple regions of the 2D-PI-BDPA sample. In general, the extremely low dose rate of 0.03 e-/Å2/s used in diffraction mode still resulted in diffraction patterns with sharp features for both high-intensity and weak spots. Figure 2a shows a SAED pattern from the first set of data collected, and diffraction spots corresponding to <2 Å resolution are observed. (Figure 2a).
![Figure 2. a) SAED pattern of 2D-PI-BPDA showing diffraction spots with resolution beyond 2Å at [001] zone axis. b) HRTEM image of the same specimen area where the SAED pattern was collected. c) Zoomed-in view of the region highlighted in 2b. d) FFT of the inset region. The total image dose is marked in a) and b).](/sites/default/files/Applications/AN_MetroLowkV/Fig%202_dose.png)
A higher beam dose of 0.09 e-/Å2/s was required to take a better signal-to-noise ratio image of the 2D-PI-BPDA lattice structure from the same exact specimen area (Figure 2b). The total dose of this image was less than 1 e-/Å2. There are variations in the lattice image contrast over the field of view, but the expected 4-fold symmetry of the 2D-PI-BPDA unit cell can be seen in the middle of the imaged area (Figure 2c). The FFT of the inset area (Figure 2d) shows the same 4-fold symmetry of the lattice structure and spots corresponding to a resolution beyond 2.3 Å. The ability to see information beyond this limit suggests that the information transfer was not limited by specimen damage but by the chosen microscope magnification and pixel size. It is expected that TEM imaging at a higher magnification, giving a smaller pixel size, would show the same 1.9 Å resolution seen in the diffraction pattern.
A beam dose rate of 6.18 e-/Å2/s was used in a different specimen area to collect an even higher contrast image of the 2D-PI-BPDA lattice. Figure 3a shows this lattice image, collected with a total dose of 31.1 e-/Å2. The defocus of the sample was slightly different from the sample region in Figure 2 and very nicely shows the approximately cubic unit cell. Figure 2a shows the raw data collected with Metro. No image filtering or image averaging was required to see the detailed lattice structure. The FFT of this area again shows information beyond 2.3 Å.
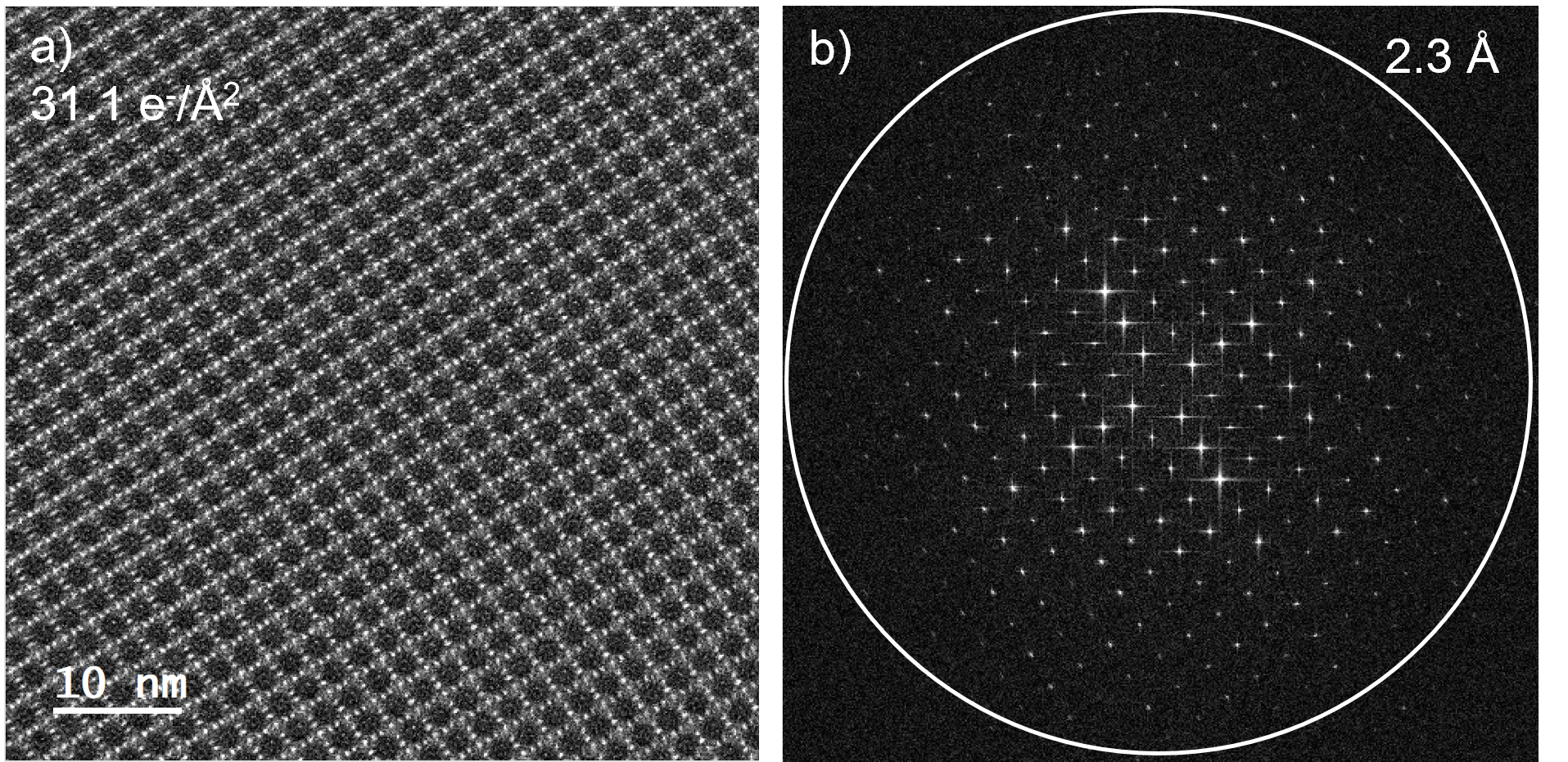
The Metro camera’s live drift correction capabilities were also critical to collecting the HRTEM images in Figures 2b,c, and Figure 3a. The extremely low dose beam conditions meant that 5 – 10 second acquisitions were needed to get a sufficient signal in the image. Given the long acquisition time and the high magnification, any minor sample drift would have affected the image quality and information transfer. Furthermore, attempting to visually assess the sample drift before capturing an image would only needlessly irradiate the sample. The live drift correction removes these complications and yields high-quality images over the long acquisition time and at low dose conditions. The visual clarity of the images in Figures 2b,c, and Figure 3a is evidence of the effectiveness of the live drift correction and their corresponding FFTs, which show even information transfer in all directions.
The highest-resolution information achieved from the investigation of 2D-PI-BPDA was 1.5 Å, as seen in Figure 4. This pattern was acquired from a third sample region using a dose rate of 0.07 e-/Å2/s. These high-resolution features were observed to quickly fade as the beam dose was increased to capture a HRTEM image. It is estimated that the spots fade with <30 e-/Å2 total dose.
![Figure 4. [001] SAED pattern of 2D-PI-BPDA showing 1.5 Å resolution diffraction spots.](/sites/default/files/Applications/AN_MetroLowkV/Fig4.png)
Comparison with previously reported results
Previous reports [1] emphasized the need for better than 2 Å resolution to fully characterize the structure of 2D-PI-BPDA. The data collected here is an excellent result that meets this criterion. The previous study used a Gatan UltraScan scintillator-based CCD camera, which does not have the high framerate and high sensitivity found in a direct detection electron counting camera like Metro. The researchers collected similar quality data through the development of a very detailed sample imaging workflow, an aberration-corrected microscope, and a lot of expertise. Furthermore, they generally could only collect a single image from a given sample area before significant sample damage.
In contrast, the sensitivity of the Metro camera and easy-to-use interface made this extremely low-dose experiment much more straightforward. Using an extremely low dose rate for searching for the sample area and acquiring data meant that the sample was minimally damaged, as seen in Figures 2 and 4. Depending on the dose rate used when imaging, multiple images could be collected from the same sample area before significantly damaging the sample. This allows for more time to adjust the image defocus and correct astigmatism.
Dose measurement and total accumulated dose per dataset
The Metro camera’s electron counting capabilities improve the sensitivity of the camera and simultaneously provide the user with a dose rate measurement. The DigitalMicrograph software displays the dose rate in e-/pixel/s and e-/Å2/s while the camera is viewing or acquiring data. When the acquisition is complete, a measurement of the total dose in e-/Å2 is shown. The acquired data is automatically saved with all three dose measurements. This makes it easy to quickly set up and control the beam dose rate used in any experiment in a reproducible and quantitative way.
Since previous reports showed that 2D-PI-BPDA begins to degrade significantly after ~85 e-/Å2 total dose, the data collection workflow outlined in the methods section was developed to stay within this total electron dose ‘budget.’ The dose measurements from the Metro camera were crucial throughout the experiment. The 85 e-/Å2 total dose budget includes the time and electrons required to find a sample area, tilt to the zone axis, and then capture all required data. The dose rates used to capture the data in Figures 2 – 4 were chosen specifically to allow enough time to set up the imaging and diffraction conditions and acquire data using both techniques. Table 1 below shows an example of the dose rates and total accumulated dose for each step of the data acquisition process.
Table 1: Utilization of electron dose budget through data collection with the Metro camera.
| Action |
Time
(s)
|
Dose Rate
(e-/Å2/s)
|
Dose
(e-/Å2)
|
Total Accumulated Dose
(e-/Å2)
|
| Find area and tilt to zone axis | ~120 | 0.07 | 8.4 | 8.4 |
| Capture 10 s SAEDP | 10 | 0.07 | 0.7 | 9.1 |
|
Capture 10 s image #1
(same dose as diffraction mode)
|
10 | 0.07 | 0.7 | 9.8 |
| Increase the dose and quickly adjust defocus | 10 | 6.2 | 62.0 | 71.8 |
|
Capture 5 s image #2
(higher dose)
|
5 | 6.2 | 31.0 | 102.8 |
Calculating the accumulated dose rates for each step reveals that utilizing dose rates <1 e-/Å2/s allows ample time to tilt the sample to the zone axis and set up the diffraction pattern acquisition after finding a good sample region. Many diffraction patterns and images could be acquired at this dose rate without coming close to the 85 e-/Å2 total accumulated dose threshold. However, capturing images with very high signal-to-noise ratios, as in Figure 3a, required dose rates on the order of ~5 e-/Å2/s. The total dose rate adds up quickly at these dose rates, so the microscopist must work quickly to set the defocus and adjust the stigmation under these conditions. Only one long-exposure image can likely be acquired at this dose rate before significantly damaging the sample. Alternatively, 2 – 3 shorter acquisitions can be done if the signal-to-noise at the given dose rate is acceptable.
Summary
The Metro camera analyzed the beam-sensitive 2D polymer material 2D-PI-BDPA under extremely low beam dose rates. Selected area electron diffraction patterns and HRTEM images were routinely collected with better than 2 Å resolution while using total accumulated doses under the threshold of 85 e-/Å2 where the sample sees significant degradation.
The direct detection electron counting capabilities of Metro are what enable data capture at dose rates <1 e-/Å2/s and provide live dose measurements to the user to guide experimental setup and data acquisition. Moreover, the workflow outlined for capturing diffraction and imaging information at extremely low beam doses can be applied to all types of beam-sensitive materials. Lower keV TEM work can also be done with Metro, which supports 60 – 200 keV operation.
Experiments that are extremely difficult, if not impossible, with a scintillator-based camera are made much more straightforward with Metro.
References
[1] Liang, B., Zhang, Y., Leist, C. et al. Optimal acceleration voltage for near-atomic resolution imaging of layer-stacked 2D polymer thin films. Nat Commun 13, 3948 (2022). https://doi.org/10.1038/s41467-022-31688-4
[2] Liu, K., Qi, H., Dong, R., Shivhare, R,. Addicoat, A., Zhang, T., Sahabudeen, H., Heine, T., Mannsfeld, S., Kaiser, U., Zheng, Z., and Feng, X,. On-water surface synthesis of crystalline, few-layer two-dimensional polymers assisted by surfactant monolayers. Nature Chemistry, 11(11):994–1000, 2019. https://doi.org/10.1038/s41557-019-0327-5
Acknowledgment
The study of [1] is funded by Deutsche Forschungsgemeinschaft (German Research Foundation) – 492191310; 426572620; 417590517 (SFB-1415).