Acquiring counted electron diffraction data without a beam stop with Gatan electron counting direct detectors
Direct detection cameras offer several advantages over scintillator-based fiber optic cameras for electron diffraction. In electron counting mode, Gatan's direct detection cameras can detect individual electrons providing a superior signal-to-noise ratio (SNR) and sharper diffraction peaks. This application note demonstrates the excellent diffraction capabilities of the Metro® and K3® cameras and describes best practices for capturing high-quality electron diffraction data with these counting cameras.
This application note demonstrates the excellent diffraction capabilities of the Metro and K3 cameras and describes best practices for capturing high-quality electron diffraction data with these detectors
Types of diffraction
There are many ways to configure a transmission electron microscope (TEM) to collect electron diffraction data. We describe four of these here, with example data from the Metro and K3 cameras.
Selected area electron diffraction
The electron beam is set as parallel as possible to collect a selected area diffraction pattern. Then a selected area aperture is used to define the region of the sample from which to form the diffraction pattern. The microscope is set to diffraction mode so that the projector lenses form an image of the back focal plane on the camera. Some correction of the diffraction focus may be necessary to make the spots as small as possible. This guide describes all the steps for setting up a TEM for selected area electron diffraction but focuses on parameters important for getting the best data from a counting camera. For more details on how to set up the TEM for selected area diffraction, see this Microscopy Today article or the Transmission Electron Microscopy book by Williams and Carter.
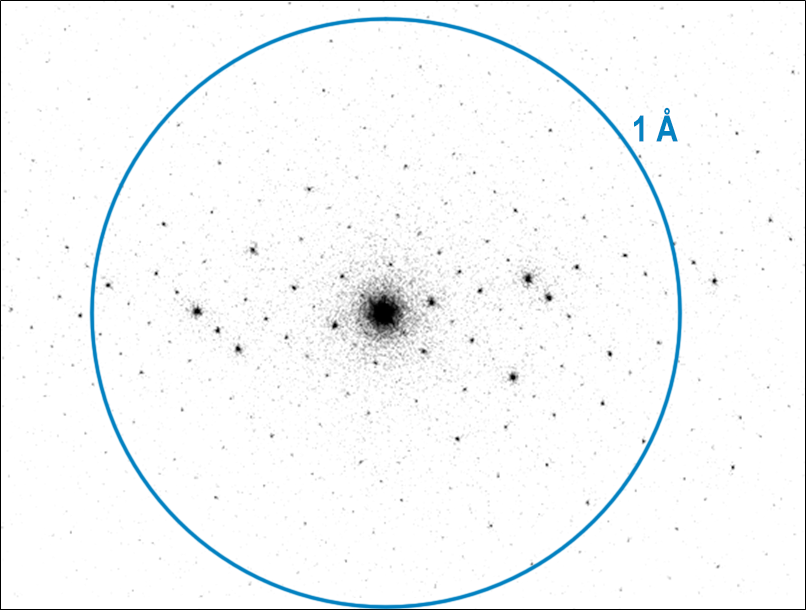
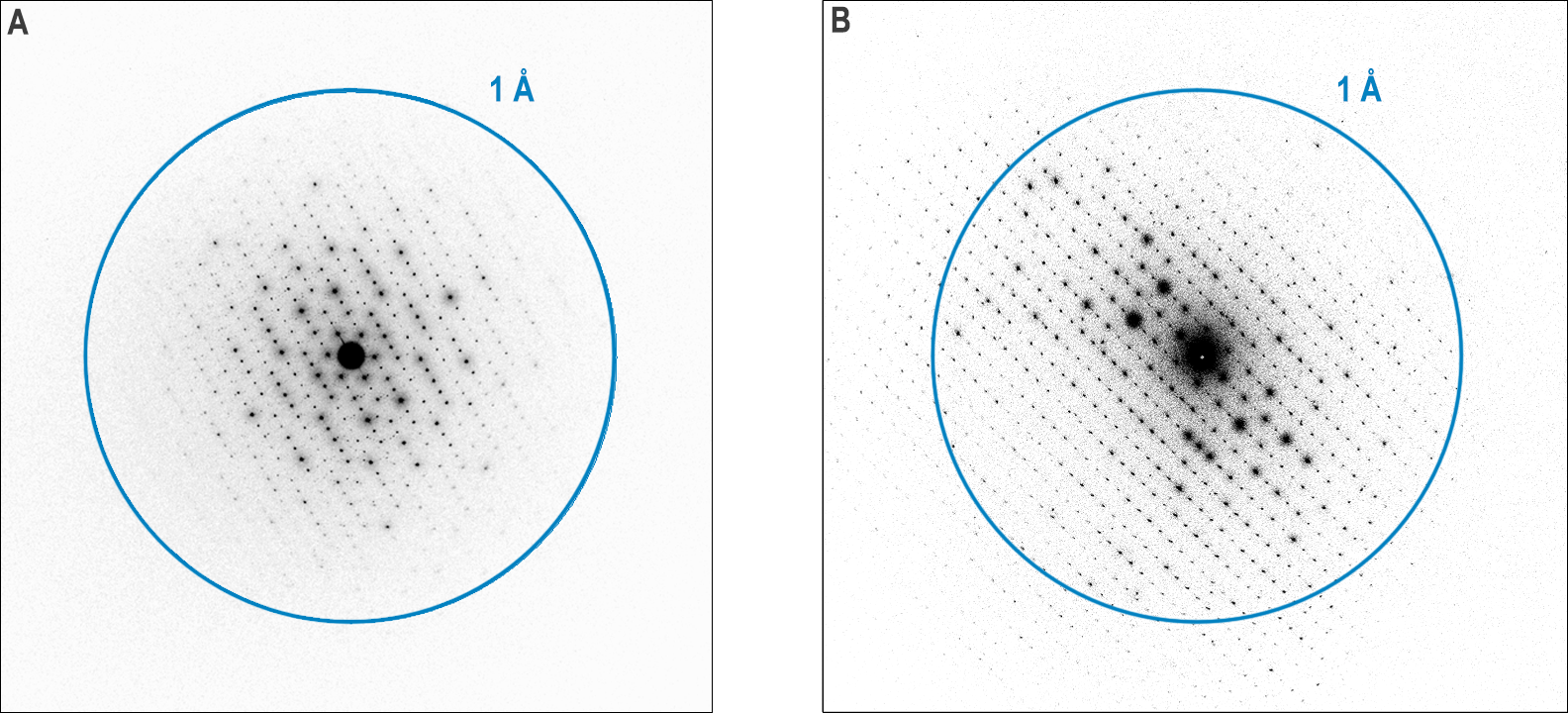
The diffraction patterns shown in Figure 1 nicely demonstrate some of the diffraction capabilities of the Metro and K3 cameras. They are both selected area electron diffraction (SAED) patterns from a ZSM-5 zeolite sample. The patterns are displayed with inverted contrast for both cameras. The SAED pattern for the Metro camera is displayed on a log scale to improve the visibility of faint spots while reducing clipping of the highest intensities. The center spot is too intense for the counting algorithm to count all the electrons, so the intensity there is not quantitative. We cover this in more detail in the Setting up SAED section. However, the center position of the pattern is visible and well-defined since there is no beam stop blocking it.
The diffraction patterns shown in Figure 1 nicely demonstrate some of the diffraction capabilities of the Metro and K3 cameras
Step-by-step directions for SAED
Continuous diffraction tomography (also known as MicroED and 3DED)
MicroED, also known as 3DED, is a technique used to determine the high-resolution structure of chemical compounds and biological macromolecules by placing the sample crystal in a TEM and continuously tilting the stage while collecting SAED data simultaneously. For more information on MicroED/3DED, refer to these papers from Structure, Frontiers in Molecular Biosciences, and eLife.
SNR and a camera frame rate high enough to record video (>30 fps) play a critical role during MicroED data collection. High SNR enables accurate amplitude measurement of Bragg intensities, particularly the high-resolution frequencies with lower amplitudes. A high camera frame rate is critical for continuous rotation during MicroED data collection. A continuous rotation is preferred to reduce the dynamic scattering caused by the interaction of the electron beam with the sample crystal and to increase sampling of the reciprocal space leading to better data completeness from a single crystal. Cameras like the K3 and Metro allow diffraction data collection without a beam stop. Figure 2 shows a projection of a MicroED dataset collected on the K3 camera.
Step-by-step instructions for MicroED
Convergent beam electron diffraction
To collect a convergent beam electron diffraction (CBED) pattern, like the one shown in Figure 3, the electron beam is set to have a large convergence angle so that the area illuminated by the beam is small and electrons pass through the sample at a wide range of angles. This can be accomplished by setting the microscope to scanning (S)TEM mode and keeping the probe in a single position or by appropriately setting up the beam convergence in some other way. Convergent beam patterns have a wealth of information, which this application note does not describe. See the Transmission Electron Microscopy book by Williams and Carter for more details.
Step-by-step instructions for CBED
4D STEM
4D STEM is a term that encompasses many different specific methods and microscope setups, all of which scan the electron beam over a sample while collecting a diffraction pattern at each scanned position. DigitalMicrograph® software achieves this in the same way that we collect electron energy loss spectroscopy (EELS) or energy dispersive x-ray spectroscopy (EDS) spectrum images, using a DigiScan™ to control the beam scanning and synchronizing the scan with the camera using fast hardware synchronization. STEMx® enables fast hardware synchronization of any camera Gatan currently produces with DigiScan's beam control.
STEMx enables fast hardware synchronization of any camera Gatan currently produces with DigiScan's beam control
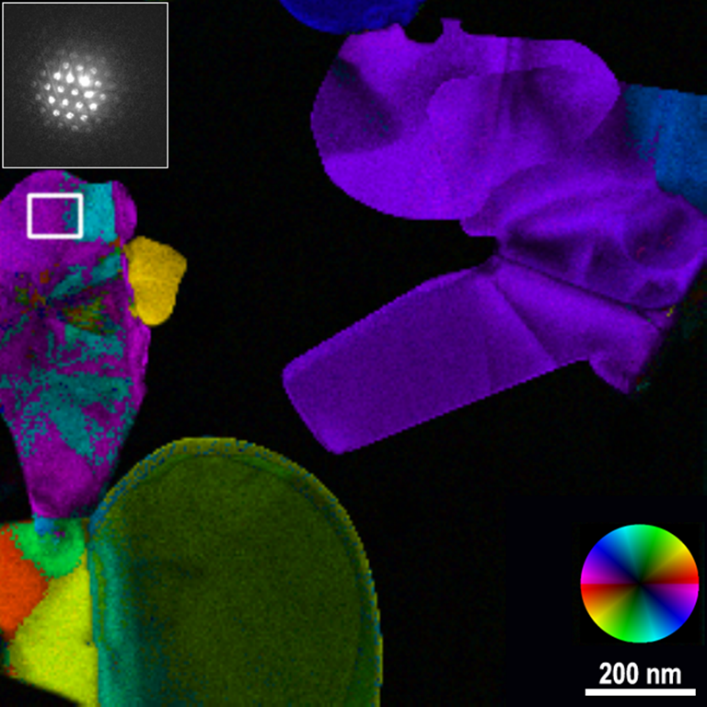
An example of a 4D STEM dataset processed via a Python script is shown in Figure 4. Due to the multi-dimensional nature of the data, it is always necessary to perform some processing before visualizing the 4D dataset in two dimensions on a screen. For more information about 4D STEM and data processing in DigitalMicrograph, see our Techniques page and this experiment brief describing the processing applied in Figure 4.
Setting up dose rate for diffraction on the Metro and K3 cameras
Considerations for diffraction on counting direct detection cameras
Properly setting up the electron dose rate for diffraction is vital to achieving the best results with any camera. There are two important hardware parameters to consider when setting up the dose rate for diffraction. The first is the maximum dose rate that can be accurately quantified. The second is the maximum dose rate that can be used without causing minor temporary damage to the sensor. Both prevent the collection of excellent diffraction data, but both should be understood when setting up the system for optimal data collection.
There are two important hardware parameters to consider when setting up the dose rate for diffraction. The first is the maximum dose rate that can be accurately quantified. The second is the maximum dose rate that can be used without causing minor temporary damage to the sensor.
Quantifying peak intensities
Metro and K3 are direct detection cameras that count each incoming electron one by one to form an image. To count all electrons without missing any, it is vital that only 1 electron hit any given pixel between subsequent sensor readouts. So, more electrons can be counted if the sensor is read out faster. Both Metro and K3 camera sensors are read at high speeds to enable fast counting of electrons. However, there are still limits on the maximum electron dose rates that can be accurately quantified. The recommended maximum dose rate for the Metro camera in D mode is 80 e-/pix/s, while the recommended maximum for the K3 is 40 e-/pix/s.
The recommended maximum dose rate for the Metro camera in D mode is 80 e-/pix/s, while the recommended maximum for the K3 is 40 e-/pix/s
Intensities much higher than the recommended maxima can be captured without damage to the sensor, as seen in the patterns shown in Figure 8 and Figure 9, but the intensities will be increasingly non-linear above 80 e-/pix/s. Suppose you need quantitative intensities or ratios of intensities of diffraction peaks for analysis. In that case, care should be taken to measure the intensities of the brightest peaks with the live view before acquisition. This can be done by placing a very small ROI over the pixels comprising the peak and reading the first value displayed by the dose rate monitor at the bottom of the screen (in e-/px/s) to ensure it is within the recommended range (<80 e-/pix/s for Metro and <40 e-/pix/s for K3 camera). Note that the first number in the dose rate monitor displays the dose per image pixel, not per physical pixel. So, for example, if Metro is set to 2x binning, then the equivalent maximum dose rate would be 4*80 = 320 e-/pix/s.
An obvious effect of this non-linear behavior is that you can observe contrast reversals when the intensity becomes large enough. In the SAED patterns from Metro and K3 shown in Figure 1, this occurs above 250 e-/pix/s and 120 e-/pix/s, respectively, about three times higher than the recommended maxima for quantitative intensities. If the acquisition conditions are set up correctly and the sample is thin, this should only be observed for the central spot, which may be orders of magnitude more intense than any of the diffraction spots. Often this contrast reversal in the central spot will be accompanied by a few bright pixels exactly in the center of the diffraction pattern, as seen in Figure 5. In practice, this contrast reversal and bright pixels make it easier to focus the diffraction pattern precisely before acquisition and to determine the pattern center precisely after acquisition. A beam-stop could be used to block the central spot, but this would make it more difficult to precisely find the center of the diffraction pattern.
In practice, this contrast reversal and bright pixels make it easier to focus the diffraction pattern precisely before acquisition and to determine the pattern center precisely after acquisition
Preventing temporary damage
The other factor to consider when setting up the dose rate on the camera is the maximum dose rate that the camera can handle without sustaining temporary burn spots in subsequent images. Both the K3 and Metro cameras can handle about 15,000 and 30,000 e-/pix/s, respectively, without any damage, which is orders of magnitude more intense than they can quantitatively measure. For a very thin and weakly scattering sample where the central spot in the diffraction pattern contains most of the electrons, the total dose rate reaching the camera should be <250,000 for K3 or <500,000 e-/s for Metro.
The total dose rate reaching the camera should be <250,000 for K3 or <500,000 e-/s for Metro
At this dose rate, if all the intensity is in the central spot, and this is focused within a 4 x 4-pixel area on the camera, each pixel will have an average of approximately 15,000 or 30,000 e-/pix/s, respectively. For thicker specimens where much of the beam intensity is scattered out of the central spot, the total dose rate can be higher than 250,000 or 500,000 e-/s since this dose rate will be distributed over a larger number of spots and camera pixels.
For thicker specimens where much of the beam intensity is scattered out of the central spot, the total dose rate can be higher than 250,000 or 500,000 e-/s...
With the direct beam or central spot of the diffraction pattern focused to a small spot directly on the camera sensor, there is always a possibility to exceed this and cause minor damage to the sensor. The first important point to keep in mind is that if the camera senses an extremely intense beam, the camera immediately blanks the beam and automatically retracts.
If the camera senses an extremely intense beam, the camera immediately blanks the beam and automatically retracts
This is known as dynamic sensor protection and will prevent serious damage to the camera. However, even with less intense beams, the sensor may sustain minor temporary damage, resulting in a subtle change to the characteristics of some pixels, which causes dark spots to appear in subsequent images. The diffraction peaks can be kept below 15k or 30k e-/pix/s, and burn spots can be avoided by practicing some simple steps to set up the diffraction conditions with the microscope. These steps are described in the Setting up SAED section below. Setting the beam up this way also minimizes damage to the sample. See Resolving burn spots in the images below for more information on mitigating and removing burn spots if they occur.
One key difference between modern CMOS cameras like Metro and K3 and previous generation CCD cameras is that Metro and K3 camera sensors are always being read out internally at a fast rate. So, when a user specifies a long exposure time, this is the sum of many short exposures, and the sensor readout rate is unchanged. This means that if the sensor is not saturated or damaged by the electron beam during a short exposure time, it will not be damaged if the user specifies a very long exposure time.
If the sensor is not saturated or damaged by the electron beam during a short exposure time, it will not be damaged if the user specifies a very long exposure time
Thus, 10 s or even 1-minute exposures are fine if the beam and sample are stable during this time, so the pattern does not change during the long exposure.
Setting up SAED
Selected area electron diffraction, where the beam is set up to be parallel, so diffraction spots are sharp points, is most challenging for a counting camera but, as demonstrated by Figure 1, Figure 8, and Figure 9, is certainly possible with the right microscope setup.
Selected area electron diffraction, where the beam is set up to be parallel, so diffraction spots are sharp points, is most challenging for a counting camera but, as demonstrated by Figure 1, Figure 8, and Figure 9, is certainly possible with the right microscope setup
This guide does not describe all the steps for setting up a TEM for selected area electron diffraction but focuses on parameters important for getting the best data from a counting camera. For more on how to set up the TEM for selected area diffraction, see this previously mentioned Microscopy Today article or Transmission Electron Microscopy by Williams and Carter.
The first key to achieving the best SAED pattern is reducing the beam's intensity
 The first key to achieving the best SAED pattern is reducing the beam's intensity. To achieve this, one could simply change the intensity/brightness setting until the beam was spread enough to achieve the desired dose rate within the selected area aperture. However, this is not ideal for beam-sensitive samples since the beam might now be irradiating (albeit faintly) a vast area of the sample. Instead, the spot size should be set, and an appropriately small condenser aperture selected so that the irradiated area can be smaller while still achieving the same low dose rate within the selected area aperture. Often a spot size of 6 – 7 for JEOL TEMs or 10 – 11 for Thermo Fisher Scientific TEMs is ideal. Note that if the spot size is pushed too far with the condenser aperture too small, it will be more difficult to maintain the alignment of the beam and selected area aperture.
The first key to achieving the best SAED pattern is reducing the beam's intensity. To achieve this, one could simply change the intensity/brightness setting until the beam was spread enough to achieve the desired dose rate within the selected area aperture. However, this is not ideal for beam-sensitive samples since the beam might now be irradiating (albeit faintly) a vast area of the sample. Instead, the spot size should be set, and an appropriately small condenser aperture selected so that the irradiated area can be smaller while still achieving the same low dose rate within the selected area aperture. Often a spot size of 6 – 7 for JEOL TEMs or 10 – 11 for Thermo Fisher Scientific TEMs is ideal. Note that if the spot size is pushed too far with the condenser aperture too small, it will be more difficult to maintain the alignment of the beam and selected area aperture.
Since the central spot intensity usually cannot be measured quantitatively in diffraction mode (as described in the section on Quantifying peak intensities), to get the best results, the beam intensity should be measured in imaging mode before switching the microscope to diffraction.
To get the best results, the beam intensity should be measured in imaging mode before switching the microscope to diffraction
With the selected area (SA) aperture inserted, the magnification should be reduced until the entire SA aperture can be seen on the camera. The SA aperture size should have already been chosen based on how large a region of the sample you want to capture diffraction from, and the magnification required depends on the aperture size.
This condition is shown in Figure 6 and Figure 7. With the full beam intensity, which will contribute to the eventual diffraction pattern hitting the camera, the brightness/intensity can be adjusted until the total dose rate on the camera is low enough. This total dose rate can be measured by multiplying the dose rate (in e/pix/s) from the dose rate monitor at the bottom of the screen by the number of pixels in the live view image to get the total number of electrons per second. For example, if the dose rate is set to 0.1 e/pix/s and the Metro camera live view image has 2048 x 2048 pixels, this means that about 419,400 electrons/s are passing through the selected area aperture, which is less than the recommended 500k e/s for the Metro camera.
This only works if the SA aperture is fully contained in the field of view of the camera, and the intensity is not too high for the camera to count accurately. Note that the dose rate within the spot will be much higher than the average dose rate on the camera, since much of the camera will be dark due to the selected area aperture. The important dose rate to measure for this calculation is the average dose rate over the entire camera, not the dose rate within the aperture.
After the beam intensity is at or below 500,000 e-/s, switch the TEM to diffraction mode and center the pattern on the camera. Adjust the camera length based on the maximum diffraction resolution you expect to observe from the sample crystal. Note that the pattern will be very faint on the phosphor screen of the TEM. Focus the central spot with the diffraction focus (not the brightness/intensity knob) while viewing the pattern on the camera and try to minimize the size of the bright region within the contrast reversal in the center of the pattern, as seen in Figure 5. If this workflow is followed, the central spot will likely have contrast reversals, as described in the section on Quantifying peak intensities. Again, this is not an indication of damage to the sensor; instead, this shows that the counting algorithm cannot keep up with the number of electrons striking the camera in each second at the very bright spots. When capturing single diffraction patterns with a low dose rate, longer exposures may be needed to achieve a good signal-to-noise ratio for faint peaks.
As explained in the section on Quantifying peak intensities, this is not a problem for counting cameras like the K3 and Metro. The ideal image capture exposure time will be defined by your experiment, not by limitations on the maximum time imposed by the camera. If acquiring MicroED or in-situ diffraction videos, you can collect data at 2 – 40 fps. The ideal tilt range and tilt speed for MicroED depend on the crystal lattice's sample type and symmetry, but a rate of 0.1 – 2º is typical.
When capturing single diffraction patterns with a low dose rate, longer exposures may be necessary to achieve a good signal-to-noise ratio for faint peaks
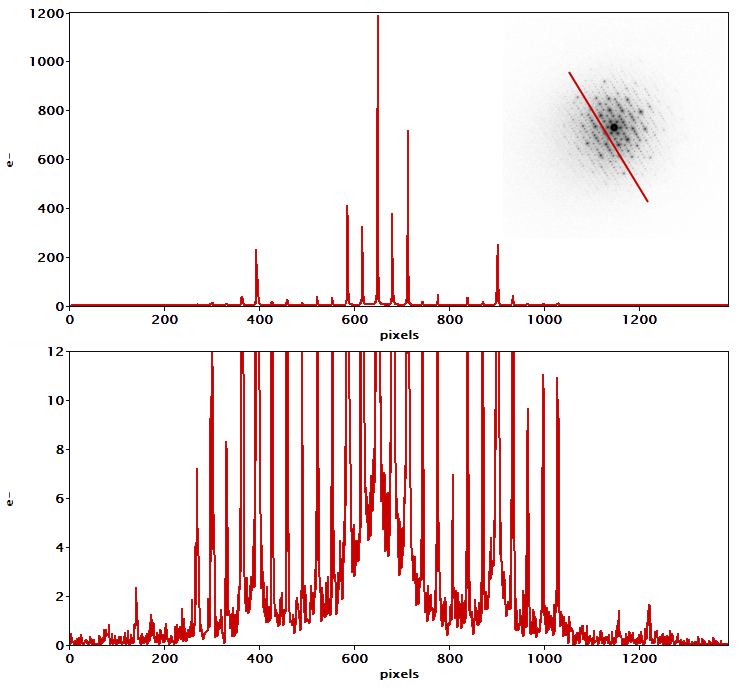
The diffraction pattern shown in Figure 8 and Figure 1a nicely demonstrates some of the diffraction capabilities of the Metro camera. The individual spots are quite sharp, with a full width at half-max (FWHM) of about two pixels for the most intense peaks shown in Figure 8. The center spot is too intense for the counting algorithm to count all the electrons, so the intensity there is not quantitative. Since the positions and relative strengths of the diffracted peaks are typically the most important, measuring accurate intensities may not be very important. The center position of the pattern is visible and well-defined since there is no beam stop blocking it.
Since the positions and relative strengths of the diffracted peaks are typically the most important, measuring accurate intensities may not be very important
The pattern in Figure 8 and Figure 1a was collected with the Metro camera set to D mode, which is optimized for diffraction. In D mode, the camera can count more electrons per second, and background noise is especially suppressed, producing patterns with a dynamic range spanning several orders of magnitude, as demonstrated by the profiles in Figure 8. Each pattern's full dynamic range and signal-to-noise ratio will be determined by the exposure time, with longer exposures leading to higher accumulated dynamic range and SNR.
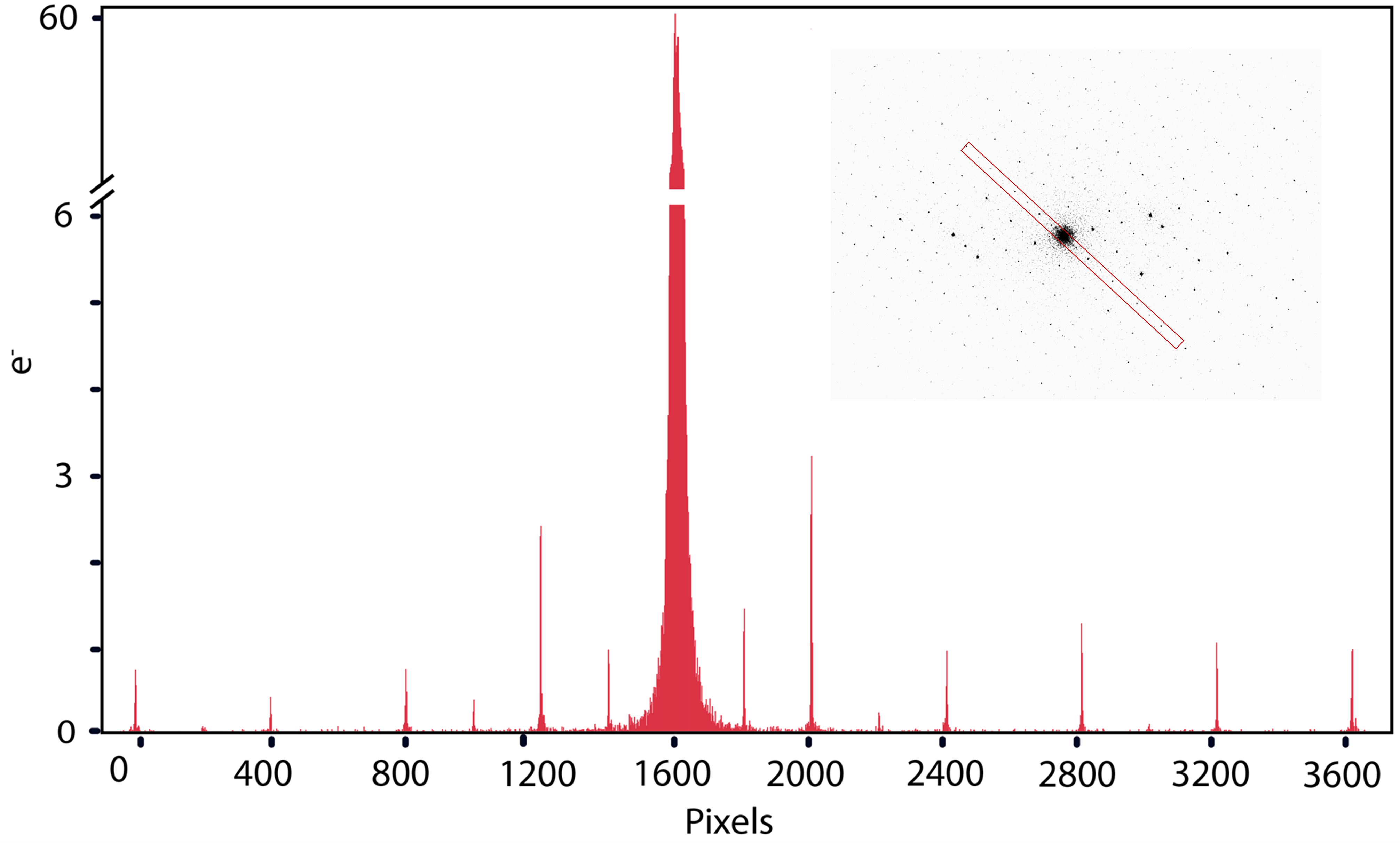
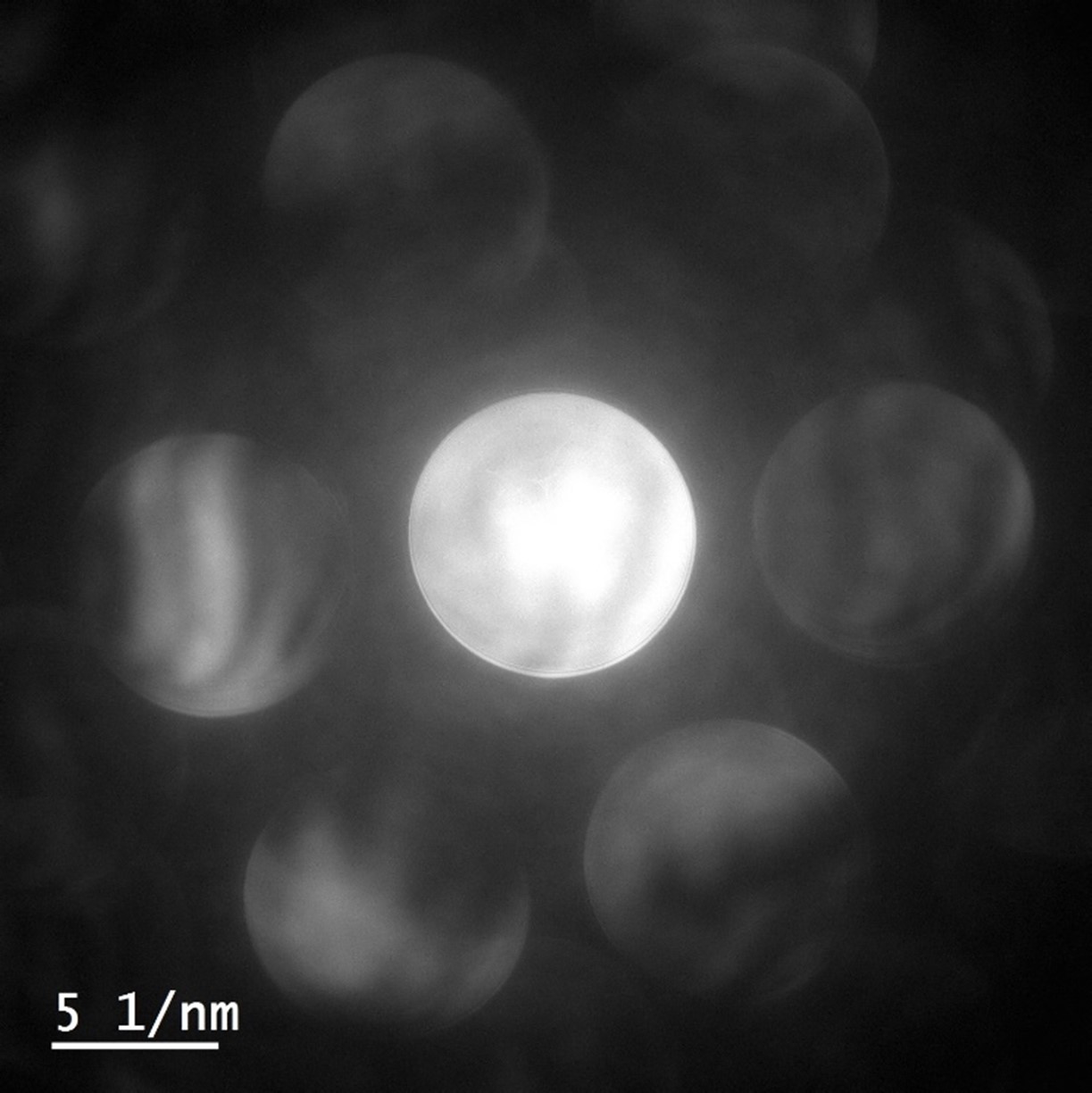
Figure 9 shows a representative MicroED projection from a ZSM-5 crystal acquired using a K3 camera with a dose of 0.002 e-/Å2/s. The diffraction pattern displays an exceptionally low background and high SNR for the Bragg intensities varying over three orders in magnitude (Figure 9). Most intensities display FWHM of about 2 – 3 pixels, and high SNR displays the advantage of electron counting for SAED and MicroED applications. Recently, Clabbers et al. reported structures of lysozyme and Proteinase K resolved at 1.2 and 1.7 Å, respectively, using MicroED on a K3 camera without a beam stop (Figure 10).
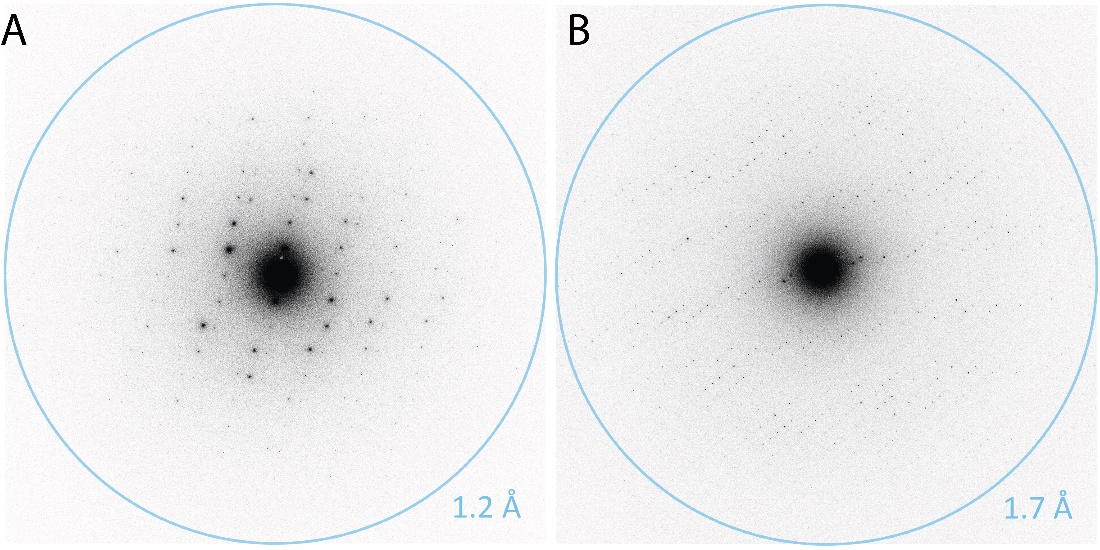
The authors used a dose rate of 0.0025 e-/Å2/s, a rotation speed of 0.15 degrees/s for data collection, and a total dose of 1 – 1.4 e-/Å2. Movies were collected over 420 – 560 s at either 2 or 10 counted frames per second (Figure 10).
Setting up CBED
This guide does not describe in detail how to set up a TEM for convergent beam electron diffraction but focuses on parameters important for getting the best data from a counting camera. For more details on how to set up the TEM for CBED, see the previously mentioned Microscopy Today article or Transmission Electron Microscopy by Williams and Carter. Unlike SAED, CBED is unlikely to focus huge beam currents into a few pixels on the camera, so contrast reversals are less likely; however, ensuring that the intensity in each diffracted spot is quantifiable and linear is often important. Thus, it is important to measure the dose rate within bright regions of the diffraction spots to ensure that it is below the maximum recommended dose rate of 80 e-/pix/s for the Metro (in D mode) and <40 e-/pix/s for the K3 camera.
CBED is unlikely to focus huge beam currents into a few pixels on the camera, so contrast reversals are less likely; however, ensuring that the intensity in each diffracted spot is quantifiable and linear is often important
If the beam intensity is too high, select a spot size that provides a lower total beam current. You may be able to increase the camera length to spread the spots over more pixels, but the desired field of view may limit this in reciprocal space. Finally, you may be able to lower the extraction voltage, though this may require discussion with the manager of the microscope.
Note that the recommended maximum dose rate assumes the images are not binned. If the images are binned, then the maximum dose rate for the Metro or K3 increases accordingly (e.g., for 1k x 1k patterns on the Metro, the maximum dose rate per pixel is 4x higher: 320 e-/pix/s). Measuring this dose rate within the spots can be achieved by placing a rectangular (not circular) ROI on the image fully within the spot and looking at the number displayed in the dose rate monitor at the bottom of the DigitalMicrograph software.
Resolving burn spots in images
If you follow the steps for setting up the camera outlined in the previous sections, burn spots should never appear. However, suppose a burn spot appears in images captured with the camera. In that case, this is likely easily remedied; it does not indicate that the camera requires servicing or sensor replacement. Instead, it indicates minor modifications to the sensor material of some pixels, which can be mitigated and even reversed, as described here.
If you follow the steps for setting up the camera outlined in the previous sections, burn spots should never appear. However, suppose a burn spot appears in images captured with the camera. In that case, this is likely easily remedied...
The first step is to acquire a new dark reference when you observe burn spots. This should temporarily remove the spots in most cases, and images with this correction are just as valid as before. However, as the sensor material slowly returns closer to its original state, the validity of the new dark reference will diminish over time (minutes to hours). Therefore, dark references may need to be acquired frequently to maintain the best performance. Fortunately, capturing a new dark reference only takes a few seconds. To capture a new dark reference, select Prepare Dark Reference from the Camera menu in DigitalMicrograph. The beam is automatically blanked during the dark reference acquisition. Note that the Camera menu is only visible in the Power User mode. Please refer to Metro and K3 user manuals for details.
The first step is to acquire a new dark reference when you observe burn spots
To permanently remove traces of burn spots from the camera, an annealing cycle should be run overnight to heat the sensor and anneal accumulated minor damage out of the sensor material (see the Metro and K3 user manuals for more details on how to run an annealing cycle). After each annealing cycle, you should acquire new gain references. Please refer to user manuals for instructions on how to acquire gain references. Periodic annealing cycles are recommended for both cameras even if no visible burn spots have been produced and should be part of the normal maintenance of the cameras, as described in the user manuals.
To permanently remove traces of burn spots from the camera, an annealing cycle should be run overnight...
Expect the Metro and K3 sensors to last the entire life of the camera. They are robust against beam damage and capable of capturing diffraction patterns without a beam stop, as described in this application note. If you believe permanent damage to the sensor has occurred, and have already taken the steps outlined above, contact Gatan Service with a description of the problem and *.dm4 images demonstrating it.
Expect the Metro and K3 sensors to last the entire life of the camera. They are robust against beam damage and capable of capturing diffraction patterns without a beam stop, as described in this application note.
Conclusion
Metro and K3 cameras can collect high-quality electron diffraction data with less background noise and sharper detail than conventional scintillator-based cameras. The fast readout speeds and robust sensors enable you to use these cameras without a beam stop if the illumination conditions are properly set up, as demonstrated by the above data. This guide to setting up diffraction should enable users to understand better and put into practice the optimal conditions for acquiring high-quality electron diffraction data on Metro and K3 cameras.