Chemical Analysis
Common challenges
There is considerable interest in emerging materials due to their extreme strength, flexibility, optical transparency and thermal conductivity. To better understand the unique properties of these materials, researchers frequently study the chemical constituents, contents, distribution, and interaction of matters, as well as reveal how these attributes change over time. These chemical analyses require single-molecule detection and imaging technologies as well as the ability to survey the results in situ under extreme temperature, pressure, velocity, radiation and vacuum conditions. Useful information to identify and quantify changes in chemical properties include:
- Chemical state relative to a distinct nanostructure or nanoparticle
- Density and symmetry of chemical properties
- Stability, phase transitions and redistribution
Innovative techniques
To adequately characterize and understand the chemical properties of your materials, you must first ensure each specimen is of the highest quality to resolve the material interface and properly controlled so you manipulate it, when necessary, under environmental stimuli. Once prepared, several techniques are available to better understand the relationship between microstructure, defects and the chemical properties of materials.
|
Electron energy loss spectroscopy (EELS) Delivers atomic-scale insights into elemental composition and chemical bonding—enabling a deeper understanding of material properties to advance fundamental research and discovery. Includes energy-filtered TEM (EFTEM). Elevate your EELS at EELS.info. |
Integrates EELS, EDS, 4D STEM, and more to deliver rich, correlated insights—advancing the understanding of complex, dynamic nanoscale phenomena. |
|
Captures real-time nanoscale dynamics under controlled stimuli to uncover fundamental mechanisms and accelerate scientific discovery. |
|
Visit compositional analysis or metals and alloys learn about related applications.
Enabling results
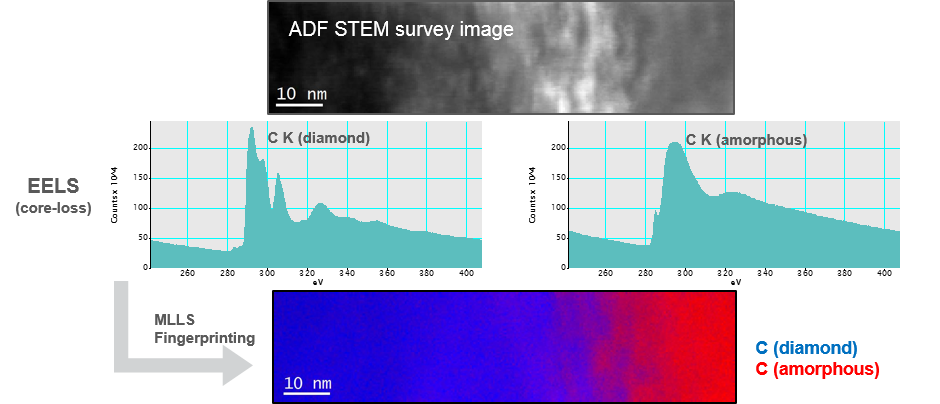
Localized chemical state
The multiple linear least squares (MLLS) fitting routine is useful to map the chemical phases within a material. When you specify reference spectra that represent the main phases present, the analysis returns fit-coefficient maps showing the spatial distribution of the reference spectra. Here you can see how this technique provides insight into the chemical state of a diamond interface after C ion implantation. Results in collaboration with and courtesy of NMMU, Port Elizabeth, South Africa.
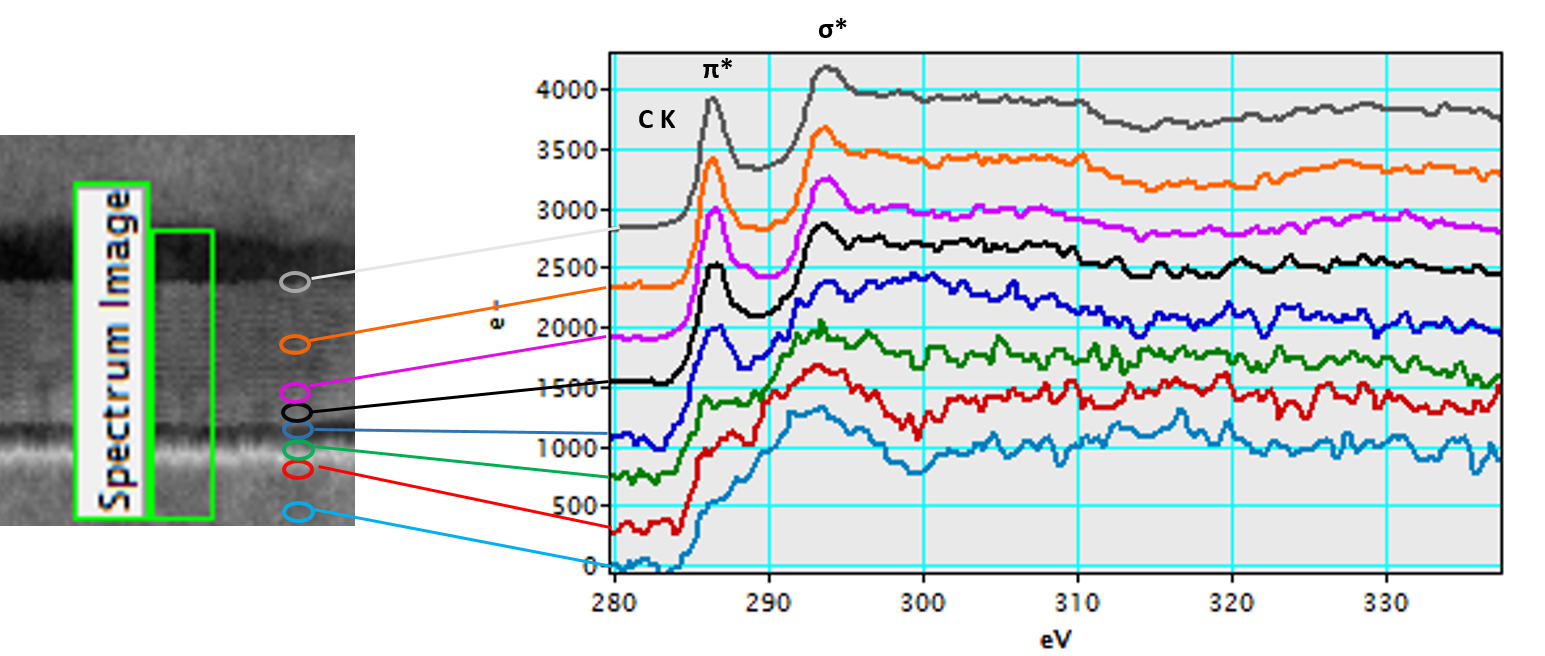
Chemical analysis
Graphene is known for its inherent strength, electrical and thermal conduction capabilities. One notable property is that graphene is the only form of carbon in which every atom is available for chemical reaction from two sides. Therefore, graphene can be more chemically reactive when you compare a single-layer to thicker sheets. Knowledge of the chemical reactivity is important when you incorporate graphene into emerging materials and devices. In this example, you can see how researchers evaluate multiple graphitic layers of an annealed sample that were exposed to high temperatures. Orange C K spectrum shows the highest π*/σ* and its peak shifted at the highest energy.